Case Report
A Case of Allergic Fungal Rhinosinusitis Associated With Abducens Nerve Palsy
Kenzo Tsuzuki*, Kengo Hashimoto, Ken Okazaki, Kazutaka Kuroda and Masafumi Sakagami
Department of Otolaryngology - Head and Neck Surgery, Hyogo College of Medicine, Japan
*Corresponding author: Kenzo Tsuzuki, Department of Otolaryngology – Head and Neck Surgery, Hyogo College of Medicine, 1-1 Mukogawa, Nishinomiya, Hyogo 663-8501, Japan
Published: 21 May, 2018
Cite this article as: Tsuzuki K, Hashimoto K, Okazaki
K, Kuroda K, Sakagami M. A Case
of Allergic Fungal Rhinosinusitis
Associated With Abducens Nerve Palsy.
Ann Clin Case Rep. 2018; 3: 1510.
Abstract
Background: Allergic Fungal Rhinosinusitis (AFRS) is characterized by Chronic Rhinosinusitis
(CRS) with nasal polyps (CRSwNP), type I and III hypersensitivity reaction, production of allergic
mucin with abundant eosinophils, and non-invading fungi. Ophthalmic complications due to
cranial neuropathies are uncommon in AFRS. A case of AFRS with abducens nerve paralysis is
reported.
Patient Description: A 66-year-old man presented with worsening diplopia and nasal obstruction.
Ophthalmologists diagnosed left abducens nerve paralysis. Edematous and polypoid mucosa with
viscous discharge was observed in the left middle and superior nasal meatuses. Blood examination
showed hyperglycemia, eosinophilia, and antigen-specific IgE positive to Aspergillus. Opacification
of a high-density area with sphenoid bony deficiency was seen on computed tomography and
iso- and low intensities were observed on T1- and T2- weighted magnetic resonance imaging in
the left maxillary, posterior ethmoid and sphenoid sinuses. Left Endoscopic Sinus Surgery (ESS)
was performed urgently under general anesthesia. Polypoid mucosa with viscous contents was
observed in the maxillary, posterior ethmoid and sphenoid sinuses during ESS. Histopathological
examination of the sphenoid sinus showed Aspergillus in the contents and marked eosinophilic
infiltration without fungal infiltration into the mucosa. Consequently, the definitive diagnosis was
AFRS. At postoperative 3 months, ophthalmologists diagnosed the diplopia as cured. Currently 6
months after surgery, there is no evidence of suspected recurrence or exacerbation.
Conclusion: AFRS should be suspected when nasal polyposis with viscous discharge and cranial
nerve paralysis are seen, and it should be carefully treated surgically.
Keywords: Allergic Fungal Rhinosinusitis (AFRS); Abducens nerve paralysis; Endoscopic Sinus
Surgery (ESS)
Introduction
Allergic Fungal Rhinosinusitis (AFRS) is characterized by Chronic Rhinosinusitis (CRS) with nasal polyps (CRSwNP), a type I (raised IgE) and possibly type III hypersensitivity reaction, production of allergic mucin with abundant eosinophils, and non-invading fungal hyphae [1]. Approximately 7% to 10% of operations for CRS are due to AFRS in the USA [2]. AFRS is thought to be refractory CRS. For a definite diagnosis, there are the classic criteria of Bent and Kuhn [3] and the clinical guidance of the American Academy of Allergy, Asthma & Immunology (AAAAI) [4]. Taken together, we diagnose AFRS when all six essential items of both criteria are fulfilled: (1) Sustained CRS symptoms for more than 12 weeks (such as nasal discharge, nasal obstruction, olfactory disorder, or facial pain); (2) Characteristic findings of rhinosinusitis on Computed Tomography (CT) or Magnetic Resonance Imaging (MRI); (3) Presence of allergic mucin on endoscopy (pathologically confirmed presence of fungi and mucosal infiltration of eosinophils); (4) Edema/polyp formation of the middle nasal meatus or middle turbinate on endoscopy; (5) Type I hypersensitivity to fungi (elevated blood serum levels of specific IgE to fungi or positive intracutaneous test); and (6) Pathologically ruled out infiltration of fungi into the surrounding mucosa. Previous studies reported that, in AFRS, ophthalmic complications due to cranial neuropathies are uncommon [5,6]. A case of AFRS that presented with diplopia due to left abducens nerve palsy is reported.
Case Presentation
A 66-year-old man presented to Hyogo College of Medicine with diplopia and nasal obstruction.
His nasal obstruction was treated by nearby ENT clinics for more than three months. Because double
vision appeared in addition to the exacerbation of nasal symptoms one month prior to his visit, he was referred to our department for treatment. On consultation
with the Department of Ophthalmology in our hospital, the double
vision was diagnosed as left abducens nerve palsy. The patient also
had diabetes mellitus.
Edematous and polypoid mucosa was observed in the left middle
nasal meatus on endoscopy (Figure 1). Around the left superior nasal
meatus and superior nasal turbinate, edematous and polypoid mucosa
with viscous discharge was also observed. No fungi were detected in
the viscous discharge on culture. No abnormality was observed in the
right nasal cavity. Blood examination showed hyperglycemia (144
mg/dl), HbA1c-NGSP (7.0%), Eosinophilia (24.4%), and Antigenspecific
IgE positive to Aspergillus (Class 2). The antigen-nonspecific
IgE level (53.3 IU/ml) was within the normal range (< 173 IU/ml).
Beta-D-glucan was also within the normal range (< 11.0 pg/ml).
CT showed opacification with a High-Density Area (HDA) in the
maxillary sinus, posterior ethmoid sinus, and sphenoid sinus on the
left side (Figure 2a). A thin bony deficiency was observed in part of the
left sphenoid bone around the superior orbital fissure (Figure 2b). T1-
and T2-weighted MRI showed iso- and low intensities, respectively,
in the left posterior ethmoid sinus, and sphenoid sinus (Figure 2c and
2d). No intracranial lesions were observed.
In order to remove and decompress the mucinous lesions
immediately, left Endoscopic Sinus Surgery (ESS) was performed
urgently under general anesthesia for the abducens nerve palsy due
to sphenoid sinusitis on the day of the initial visit to our department
in the hospital. Blood loss was less than 10 mL. Surgical findings
of the paranasal sinuses showed polypoid mucosal swelling and
viscous contents in the posterior ethmoid sinus, sphenoid sinus,
and maxillary sinus (Figure 3). Although the anterior ethmoid sinus
showed edematous mucosal swelling, there was no retention of
viscous contents. Inflammation was not observed in the frontal sinus.
Histopathological examination of the specimens of the sphenoid sinus
during ESS showed Aspergillus in the contents (Grocott stain, (Figure
4a)) and marked eosinophilic infiltration without fungal infiltration
into the sinus mucosa (Hematoxylin-Eosin (H&E) stain, (Figure 4b)). Since this case fulfilled all six essential items of the diagnostic
criteria (mentioned above), a definite diagnosis of AFRS was made.
No antifungal medicine was administered before and after surgery.
Corticosteroids (steroids) were not given systemically because of the
history of diabetes mellitus. Since the patient’s postoperative course
was uneventful, he was discharged on postoperative day 5. Three
months after ESS, the ophthalmologists declared the diplopia cured.
On endoscopy and CT, partial mucous membrane swelling remained
in the left sphenoid sinus, but no abnormality was found in other
paranasal sinuses (Figure 5). Currently, 9 months after ESS, there
have been no findings suggestive of recurrence or exacerbation.
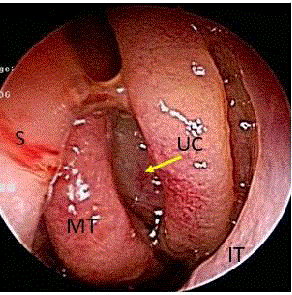
Figure 1
Figure 1
Preoperative left nasal endoscopic findings. Edematous and
polypoid mucosa is observed in the left middle nasal meatus (arrow).
MT: Middle Turbinate; IT: Inferior Turbinate; S: Nasal Septum; UC: Uncinate
Process
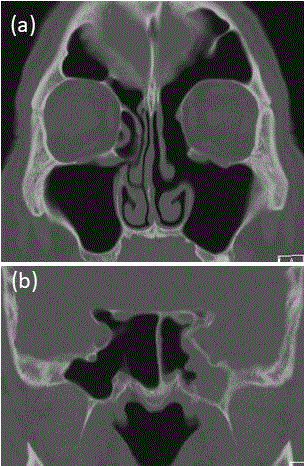
Figure 2
Figure 2
Preoperative findings on sinonasal imaging. CT (a, b) and MRI (c,
d): Arrowheads indicate the viscous effusion suggesting eosinophilic mucins
in the maxillary sinus, posterior ethmoid sinus, and sphenoid sinus on the left
side (a, c, d); the arrow indicates a thin bony deficiency in a part of the left
sphenoid bone around the superior orbital fissure (b).
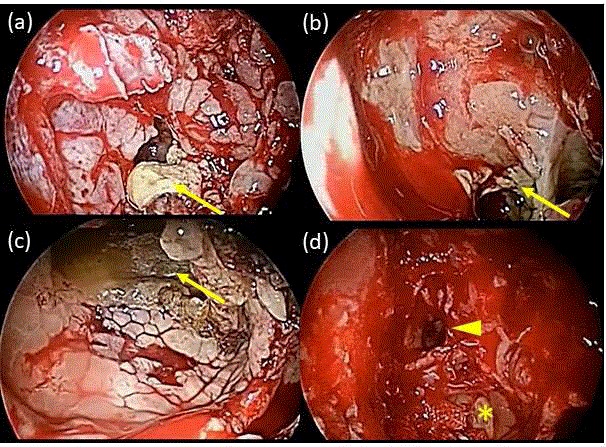
Figure 3
Figure 3
Surgical findings. The polypoid mucosal swelling and viscous
contents in the posterior ethmoid sinus (a), sphenoid sinus (b), and maxillary
sinus (c). The anterior ethmoid sinus showed edematous mucosal swelling
without viscous contents (d, asterisk). Inflammation was not observed in the
frontal sinus (d, arrowhead). Arrows indicate viscous contents.
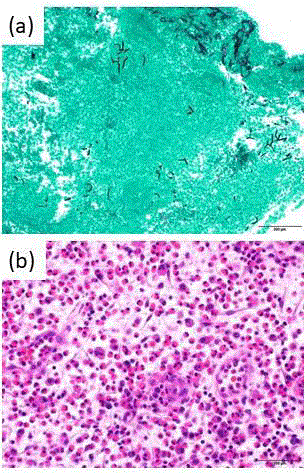
Figure 4
Figure 4
Histopathological findings in the sphenoid sinus.
(a) Grocott stain shows the presence of Aspergillus in the sphenoid sinus
contents.
(b) H&E stain shows marked eosinophilic infiltration without fungal infiltration
in the sinus mucosa. Scale bars: 200 μm.
Figure 5
Figure 5
Postoperative CT findings.
Three months after ESS with the diplopia considered cured, no abnormality
is found in the maxillary, ethmoid, and frontal sinuses (a), except for partial
mucous membrane swelling remaining in the left sphenoid sinus (b).
Discussion
A case of abducens neuropathy due to AFRS was reported,
since, to the best of our knowledge, there have been no case reports
of abducens neuropathy due to AFRS in Japan according to a
previous nationwide report [7]. A total of 37 cases of AFRS fulfilling
the diagnostic criteria of AAAAI have been reported in Japan, and
8% of them had ophthalmic symptoms (3/37 cases: 2 cases with
exophthalmos and one case with decreased vision) [7]. Although
there was a report that 3% (3/94) of AFRS cases in the USA had
abducens paralysis, its occurrence is thought to be uncommon [6].
As mechanisms causing cranial neuropathies due to rhinosinusitis,
inflammation affecting adjacent nerves and mechanical compression,
which can cause direct nerve compression and/or a circulatory
disorder due to vascular compression, can be considered. The
abducens nerve is the most common cranial nerve to be affected due
to its medial anatomical location in the cavernous sinus and proximity
to the sphenoid sinus, followed by the optic nerve [8]. In AFRS, the
volume of allergic eosinophilic inflammatory mucinous products of
fungal colonies increases gradually and their expansion causes bony
demineralized erosion and nerve compression [9]. Approximately
20% of patients with AFRS demonstrated bone erosion on CT scan
[10], and male patients were reported to be at higher risk of bone
erosion than female patients [11]. Therefore, the pathogenesis of
diplopia in the present case could be the result of inflammation of the sphenoid sinus spreading directly to the abducens nerve and
expansive compression of the abducens nerve due to eosinophilic
mucin produced by the fungi with sphenoid bone destruction.
With respect to the management of Fungal Rhinosinusitis (FRS),
accurate early diagnosis before treatment is very important, because
the medical treatment strategies for infectious and non-infectious
(AFRS) diseases differ. For infectious FRS, immune suppressors,
such as steroids, can never be used, because they can stimulate and
exacerbate fungal infiltration. In cases of invasive type, antifungal
therapy is required until serum beta-Dglu can levels decrease to
within normal limits [12]. On the other hand, for AFRS, steroids
are indicated and useful, with careful attention to Cushingoid side
effects, where as the benefits of antifungal therapy are still unclear
[13]. A combination of steroids and ESS is required, and oral steroids
can subsequently lead to short-term postoperative improvement
[13]. Although oral steroids could not be administered to the present
case with diabetes mellitus, steroids might be required for future
recurrence of AFRS.
In the differential diagnosis, Eosinophilic CRS (ECRS)is one of
the most important diseases. Based on the Japanese Epidemiological
Survey of Refractory Eosinophilic Chronic Rhinosinusitis (JESREC)
study, ECRS is definitely diagnosed by the following four items:
bilateral lesions; nasal polyps; ethmoid sinus-dominant; and
blood eosinophilia [14]. The majority of ECRS cases have bilateral
involvement, whereas less than 30% of AFRS cases show bilateral
involvement in Japan [7]. Unilateral AFRS might become bilateral
in the future. Some patients with unilateral AFRS certainly fulfill
the JESREC criteria because of CRSwNP with severe eosinophilia
(>10%), as shown in the present case. Patients with ECRS who fulfill
the diagnostic criteria for AFRS might also exist. It is important when
making the diagnosis to consider the possibility of AFRS by being
sufficiently aware of the disease, though the pathogenesis of AFRS
still remains controversial, with some uncertain issues [13].
In the present case, ESS was performed one month after the onset
of ophthalmic symptoms, and fortunately the abducens paralysis
improved. ESS is necessary in the earlier stage for rhinogenic ocular
symptoms [10]. The ocular manifestations of AFRS can be reversible
if promptly and appropriately addressed and decompression is
performed [6,10]. Fortunately, no recurrence and good progress have
been seen in the present patient. However, since AFRS is thought to
be refractory and have a high possibility of recurrence, continuous
careful long-term follow-up observation is required [15].
Conclusion
We encountered an uncommon case of AFRS that presented with abducens neuropathy, which has not been previously reported in Japan. Lessons learned from the present case are that what we should take into consideration the possibility of AFRS when we observe nasal polyposis with viscous nasal discharge and cranial nerve paralysis, and what ophthalmic symptoms should be treated surgically in the early stage.
References
- Chakrabarti A, Denning DW, FergusonBJ, Ponikau J, Buzina W, Kita H, et al. Fungal rhinosinusitis: A categorization and definitional schema addressing current controversies. Laryngoscope. 2009;119(9):1809-18.
- Ryan MW. Allergic fungal rhinosinusitis. Otolaryngol Clin North Am. 2011;44(3):697-710.
- Bent JP 3rd, Kuhn FA. Diagnosis of allergic fungal sinusitis. Otolaryngol Head Neck Surg. 1994;111(5):580-8.
- Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, et al. Rhinosinusitis: developing guidance for clinical trials. J Allergy Clin Immunol. 2006;118(5):S17-61.
- Marple BF, Gibbs SR, Newcomer MT, Mabry RL. Allergic fungal sinusitis-induced visual loss. Am J Rhinol. 1999;13(3):191-5.
- Illing EA, Dunlap Q, Woodworth BA. Outcomes of pressure-induced cranial neuropathies from allergic fungal rhinosinusitis. Otolaryngol Head Neck Surg. 2015;152(3):541-5.
- Nakatani A, Maeda Y, Hayama M, ShikinaT, Obata S, Takeda K, et al. Allergic fungal rhinosinusitis in Japan: A clinical analysis of 8 cases from our institute and a review of 29 cases reported nationwide. Nippon Jibiinkoka Gakkai Kaiho. 2017;120:1457-66.
- El Mograbi A, Soudry E. Ocular cranial nerve palsies secondary to sphenoid sinusitis. World J Otorhinolaryngol Head Neck Surg. 2017;3(1):49-53.
- Reitzen SD, Lebowitz RA, Jacobs JB. Allergic fungal sinusitis with extensive bone erosion of the clivus presenting with diplopia. J Laryngol Otol. 2009;123(7):817-9.
- Nussenbaum B, Marple BF, Schwade ND. Characteristics of bony erosion in allergic fungal rhinosinusitis. Otolaryngol Head Neck Surg. 2001;124(2):150-4.
- Ghegan MD, Wise SK, Gorham E, Schlosser RJ. Socioeconomic factors in allergic fungal rhinosinusitis with bone erosion. Am J Rhinol. 2007;21(5):560-3.
- Yamaguchi J, Kawabata T, Motomura A, Hatano N, Seki Y. Fungal internal carotid artery aneurysm treated by trapping and high-flow bypass: A case report and literature review. Neurol Med Chir. 2016;56(2):89-94.
- Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. Rhinology. 2012;50:1-12.
- Tokunaga T, Sakashita M, Haruna T, Asaka D, Takeno S, Ikeda H, et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis:the JESREC Study. Allergy. 2015;70:995-1003.
- Kuhn FA, Javer AR. Allergic fungal sinusitis: a four-year follow-up. Am J Rhinol. 2000;14(3):149-56.