Case Report
De Novo Formation and Rupture of an Intracranial Aneurysm within 17 Days in an 84-Year Old Patient without a Previous History of Intracranial Aneurysms: A Case Report
Wouter M Sluis, Gabriel JE Rinkel and Ynte M Ruigrok*
Department of Neurology and Neurosurgery, University Medical Center Utrecht, Netherlands
*Corresponding author: Ynte M Ruigrok, Department of Neurology and Neurosurgery, University Medical Center Utrecht, PO Box 85500, 3508 GA Utrecht, Netherlands
Published: 18 Nov, 2017
Cite this article as: Sluis WM, Rinkel GJE, Ruigrok YM.
De Novo Formation and Rupture of
an Intracranial Aneurysm within 17
Days in an 84-Year Old Patient without
a Previous History of Intracranial
Aneurysms: A Case Report. Ann Clin
Case Rep. 2017; 2: 1470.
Abstract
Development and rupture of an intracranial aneurysm within a short time is rare, in particular in patients without a previous history of intracranial aneurysms. Here we describe an octogenarian who died from a subarachnoid hemorrhage (SAH) from an aneurysm of the left internal carotid artery that was not present on imaging 17 days earlier. This case report shows that aneurysms can develop and rupture within weeks, even in octogenarians without a previous history of intracranial aneurysms.
Introduction
Intracranial aneurysms are not present at birth but develop during life, usually after the third decade [1,2]. Factors involved in and the pace of development of aneurysms are incompletely understood. Newly developed aneurysms at sites without an aneurysm at earlier imaging (so called de novo aneurysms) have been mainly demonstrated in patients with a known history of intracranial aneurysm who develop a second aneurysm at a different location. Most of these de novo aneurysms have been found several months or years after previous imaging [3-34]. De novo aneurysms developing and rupturing within two months are rare and have only been reported in young or middle aged patients undergoing vascular neurosurgical procedures [35-39]. We describe an octogenarian without a previous history of an intracranial aneurysm who died from a subarachnoid hemorrhage (SAH) from an aneurysm that was not present on imaging of the intracranial arteries seventeen days before the hemorrhage.
Case Presentation
A patient with a previous history of hypertension, type 2 diabetes and a myocardial infarction who has never smoked was admitted to our hospital because of a history of slowly developing left frontal headache and progressive visual loss of the left eye within several weeks. Neurological examination showed a central visual defect of both eyes with an uncorrected visual acuity of 4/10 right eye and 2/10 left eye and no other abnormalities. Laboratory blood testing showed a mildly elevated ESR (of 44 mm per hour). Magnetic resonance imaging (MRI) of the head showed a mass in the sphenoid sinus with a close relation to the left optic nerve, possibly explaining the loss of vision of the left eye but not of the right eye (Figure 1). A sphenoidectomy and biopsy of the tumor was performed, that showed signs of a chronic infection. The patient was started on 60 mg prednisone a day. One month later and still being on steroids the patient had increasing headaches and decreasing vision of the left eye to perception of light only. A CT scan showed that the sphenoid sinus mass had not progressed in size; intracranial arteries were normal (Figure 2). A second surgery was performed to remove the mass in the left sphenoid sinus. Pathology showed a poorly differentiated tumor, probably a neuro-endocrine carcinoma or esthesioneuroblastoma. A lumbar puncture showed no abnormalities. Steroid therapy was stopped. On day 17 post surgery, awaiting radiotherapy the patient suddenly lost consciousness and was transported to the hospital. On examination in the ER, the patient did not open eyes, localized on painful stimuli but had no verbal reaction (Glasgow Coma Scale E-1 M-5 V-1). CT scanning showed subarachnoid hemorrhage (Figure 3) and an aneurysm of the left internal carotid artery diagnosed by CT-angiography (Figure 4). In retrospect, this aneurysm was not seen on any of the previous images. The patient died one day after the SAH; autopsy was not permitted.
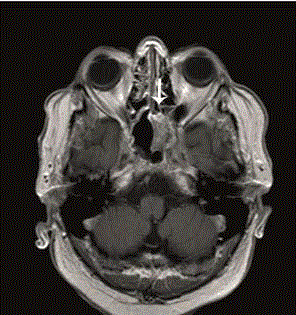
Figure 1
Figure 1
T1-MRI + gadolinium of the head showing the mass in the left
sphenoid (as indicated by the arrow).
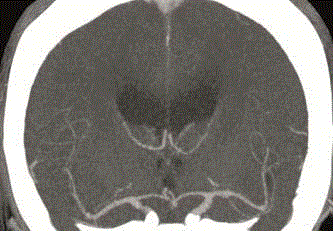
Figure 2
Figure 2
Second CT of the head with contrast enhancement performed 29
days after the first CT, with no abnormalities seen in the arteries of the Circle
of Willis.
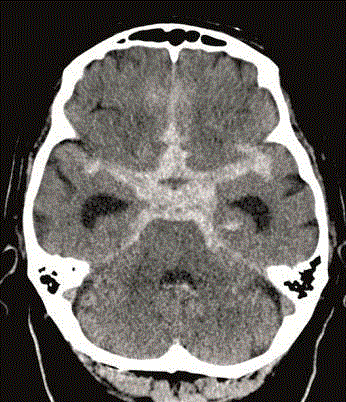
Figure 3
Figure 3
CT of the head (performed 17 days after the second CT with
contrast enhancement) showing the subarachnoid hemorrhage.
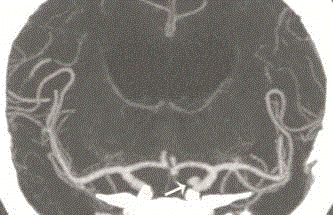
Figure 4
Figure 4
CT-Angiography of the head showing the aneurysm of the left
internal carotid artery (as indicated by the arrow).
Discussion
This patient without a previous history of intracranial aneurysms died from rupture of an aneurysm that was not present on imaging 17 days earlier. In the literature we only found one other patient without a history of aneurysms who had a SAH from an aneurysm that was not present on recent imaging, but in this patient the interval between the previous imaging and the SAH was 10 months [40]. Patients with a previous aneurysmal SAH are at risk of the development and rupture of de novo intracranial aneurysms [3,13,30]. In the literature we found five case reports of patients with a previous history of aneurysmal SAH who had a new episode of SAH from a de novo aneurysm that had developed within two months [35-39]. Most de novo aneurysms are diagnosed around the fourth to fifth decade [2,3,41], and as far as we know, no previous patients have been described with a de novo development of an aneurysm in the ninth decade, as in our patient. It is unlikely that the aneurysm in our patient developed as a result of direct injury of the carotid artery during surgery, or extension of the sphenoid mass into the subarachnoid space, because the walls of the sphenoid sinus in our patient were still intact on imaging at time of the SAH and because the aneurysm was localized at the posterior side of the carotid artery. Intracranial aneurysm is considered a complex disease characterized by a process of initiation, growth, and rupture, with multiple genetic and environmental risk factors that might contribute to one or more of the stages [42]. Aneurysms may grow fast in a short period of time and these fast growing aneurysms are likely to have high risks of rupture for short periods of time [43], which is illustrated by the case description of our patient. The case also illustrates that these processes are regardless of age. In conclusion, this case report adds to the literature that de novo aneurysms can develop rapidly without a previous history of SAH or aneurysms, even so in elderly patients.
References
- Miller CA, Hill SA, Hunt WE. "De novo" aneurysms. A clinical review. Surg Neurol. 1985; 24: 173-180.
- Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol. 2011; 10: 626-636.
- Wermer MJH, van der Schaaf IC, Rinkel GJE. Follow-up screening after subarachnoid haemorrhage: frequency and determinants of new aneurysms and enlargement of existing aneurysms. Brain. 2005; 128: 2421-2429.
- Arambepola PK, McEvoy SD, Bulsara KR. De novo aneurysm formation after carotid artery occlusion for cerebral aneurysms. Skull base. 2010; 20: 405-408.
- Akyuz M, Tuncer R, Yilmaz S. Angiographic follow-up after surgical treatment of intracranial aneurysms. Acta Neurochir (Wien). 2004; 146: 245-250.
- Bruneau M, Rynkowski M, Smida-Rynkowska K. Long-term follow-up survey reveals a high yield, up to 30% of patients presenting newly detected aneurysms more than 10 years after ruptured intracranial aneurysms clipping. Neurosurg Rev. 2011; 34: 485-496.
- Campeau NG, Lane JI. De novo development of a lesion with the appearance of a cavernous malformation adjacent to an existing developmental venous anomaly. Am J Neuroradiol. 2005; 26: 156-159.
- Chauveau D, Sirieix ME, Schillinger F, Legendre C, Grünfeld JP. Recurrent rupture of intracranial aneurysms in autosomal dominant polycystic kidney disease. BMJ. 1990; 301: 966-967.
- Edner G, Amlqvist H. The Stockholm 20-year follow-up of aneurysmal subarachnoid hemorrhage outcome. Neurosurgery. 2007; 60: 1017-1023.
- Huston J, Torres VE, Wiebers DO. Follow-up of intracranial aneurysms in autosomal dominant polycystic kidney disease by magnetic resonance angiography. J Am Soc Nephrol. 1999; 10: 2135-2141.
- Ikeda T, Kurita H, Konishi Y, Fujitsuka M, Hino K, Shiokawa Y, et al. De novo dissecting aneurysm in a patient with a ruptured saccular lesion. Case report. J Neurosurg. 2002; 97: 701-704.
- Johnston SC, Halbach VV, Smith S, Gress D. Rapid development of giant fusiform cerebral aneurysms in angiographically normal vessels. Neurology. 1998; 50: 1163-1166.
- Kemp WJ, Fulkerson DH, Payner TD, Leipzig TJ, Horner TG, Palmer EL, et al. Risk of hemorrhage from de novo cerebral aneurysms. J Neurosurg. 2013; 118: 58-62.
- Koroknay-Pál P, Niemelä M, Lehto H, Kivisaari R, Numminen J, Laakso A, et al. De novo and recurrent aneurysms in pediatric patients with cerebral aneurysms. Stroke. 2013; 44: 1436-1439.
- Kurokawa T, Harada K, Ishihara H, Fujisawa H, Kato S, Kajiwara K, et al. De novo aneurysm formation on middle cerebral artery branches adjacent to the anastomotic site of superficial temporal artery-middle cerebral artery bypass surgery in two patients: technical case report. Neurosurgery. 2007; 61: 297-298.
- Lebland R. De novo formation of familial cerebral aneurysms: case report. Neurosurgery. 1999; 44: 871-876.
- Liberman AL, Nagel MA, Hurley MC, Caprio FZ, Bernstein RA, Gilden D. Rapid development of 9 cerebral aneurysms in Varicella-Zoster virus vasculopathy. Neurology. 2014; 82: 2139-2141.
- Maiuri F, Spaziante R, Iaconetta G, Signorelli F, Cirillo S, Di Salle F. 'De novo' aneurysm formation: a report of two cases. Clin Neurol Neurosurg. 1995; 97: 233-238.
- Marchel A, Bidzinski J, Bojarski P. Formation of new aneurysms. Report of five cases. Acta Neurochir. 1991; 112: 96-99.
- Matheus M, Castillo M. Development of de novo intracranial aneurysm in three months: case report and literature review. AJNR. 2003; 24: 709-710.
- Ibrahim T, Hafez A, Raj R. De novo giant A2 aneurysm following anterior communicating artery occlusion. Surg Neurol Int. 2015; 23: 560-565.
- Misra BK, Whittle IR, Steers AJ, Sellar RJ. De novo saccular aneurysms. Neurosurgery. 1988; 23: 10-15.
- Obray R, Clatterbuck R, Olvi A. De Novo Aneurysm Formation 6 and 22 Months after Initial Presentation in Two Patients. Am J Neuroradiol. 2003; 24: 1811-1813.
- Nakajima F, Shibahara N, Arai M. Intracranial aneurysms and autosomal dominant polycystic kidney disease: followup study by magnetic resonance angiography. J Urol. 2000; 164: 311-313.
- Parekh HC, Prabhu SS, Keogh AJ. De novo development of saccular aneurysms: report of two cases. Br J Neurosurg. 1995; 9: 695-698.
- Rahmah NN, Horiuchi T, Kusano Y, Sasaki T, Hongo K. De novo Aneurysm: Case Reports and Literature Review. Neurosurgery. 2011; 69: 761-766.
- Sakaki T, Tominaga M, Miyamoto K, Tsunoda S, Hiasa Y. Clinical studies of de novo aneurysms. Neurosurgery. 1993; 32: 512-517.
- Sakaki T, Takeshima T, Tominaga M, Hashimoto H, Kawaguchi S. Recurrence of ICA-PCoA aneurysms after neck clipping. J Neurosurg. 1994; 80: 58-63.
- Tsutsumi K, Ueki K, Usui M, Kwak S, Kirino T. Risk of recurrent subarachnoid hemorrhage after complete obliteration of cerebral aneurysms. Stroke. 1998; 29: 2511-2513.
- Tsutsumi K, Ueki K, Morita A, Usui M, Kirino T. Risk of aneurysm recurrence in patients with clipped cerebral aneurysms. Results of long-term follow-up angiography. Stroke. 2001; 31: 1191-1194.
- Wermer MJ, Rinkel GJ, van Gijn J. Repeated screening for intracranial aneurysms in familial subarachnoid haemorrhage. Stroke. 2003; 34: 2788-2791.
- Yamakawa H, Sakai N, Takenaka K, Yoshimura S, Andoh T, Yamada H, et al. Clinical analysis of recurrent subarachnoid hemorrhage after neck clipping surgery. Neuro Med Chir. 1997; 37: 380-385.
- Yoneoka Y, Takeda N, Akira I, Ibuchi Y, Kumagai T, Sugai T, et al. Ruptured de novo intracranial aneurysms. Acta Neurochir. 2004; 146: 979-981.
- Zali A, Khosnood R, Zarghi A. De Novo aneurysms in long-term follow-up CT-angiography of patients with clipped intracranial aneurysms. World Neurosurg. 2014; 82: 722-725.
- Yasuhara T, Tamiya T, Ohmoto T. De novo formation and rupture of an aneurysm: Case report. J Neurosurg. 2002; 97: 697-700.
- Sim JH, Kim SC, Kim MS. Early development and rupture of de novo aneurysm: Case report. Neurol med Chir. 2002; 42: 334-337.
- Schebesch K, Doenitz C, Brawanski AT. Recurrent subarachnoid hemorrhage caused by a de novo basilar tip aneurysm developing within 8 weeks after clipping of a ruptured anterior communicating artery aneurysm: case report. Neurosurgery online. 2008; 62: 259-260.
- Ha S, Lim D, Kim S. Rupture of de novo anterior communicating artery aneurysm 8 days after the clipping of ruptured middle cerebral artery aneurysm. J Korean Neurosurg Soc. 2013; 54: 236-238.
- Wang YY, Rosenfeld JV, Lyon M. Rapid development of a de novo intracranial aneurysm following carotid occlusion. J Clin Neurosci. 2008; 15: 324-330.
- Okazaki T, Nishi T, Fujioka S. De novo formation and rupture of an intracranial aneurysm 10 months after normal findings on conventional magnetic resonance angiography in a patient with no history of intracranial lesions. Neurol Med Chir. 2010; 50: 309-312.
- Ferns SP, Sprengers ME, van Rooij WJ, van den Berg R, Velthuis BK, de Kort GA, et al. De novo aneurysm formation and growth of untreated aneurysms: a 5-year MRA follow-up in a large cohort of patients with coiled aneurysms and review of the literature. Stroke. 2011; 43: 313-318.
- Tromp G, Weinsheimer S, Ronkainen A, Kuivaniemi H. Molecular basis and genetic predisposition to intracranial aneurysm. Ann Med. 2014; 46: 597-606.
- Juvela S, Poussa K, Porras M. Factors affecting formation and growth of intracranial aneurysms: a long-term follow-up study. Stroke. 2001; 32: 485-491.