Case Report
Pregnancy in Patients with Established Liver Cirrhosis – An Uncommon but High-Risk Situation
Marcus Robertson1,2*, Andrew Bathgate1 and Peter Hayes1
1Department of Hepatology, Royal Infirmary of Edinburgh, United Kingdom
2Department of Gastroenterology, Monash Health, Melbourne, Australia
*Corresponding author: Marcus Robertson, c/ Monash Health Gastroenterology and Hepatology Unit 246 Clayton Road, Clayton, Victoria, Australia 3068
Published: 30 Aug, 2017
Cite this article as: Robertson M, Bathgate A, Hayes P.
Pregnancy in Patients with Established
Liver Cirrhosis – An Uncommon but
High-Risk Situation. Ann Clin Case
Rep. 2017; 2: 1426.
Abstract
Pregnancy is uncommon in females with liver cirrhosis but represents a complicated and highrisk clinical situation. Cirrhosis and portal hypertension may significantly worsen during pregnancy placing both the mother and foetus at risk of serious adverse events. In women with pre-existing portal hypertension, 30% - 64% will suffer from liver-related complications during or after pregnancy, most commonly manifesting as either variceal haemorrhage or hepatic decompensation, with maternal mortality rates of 1.8% - 7.8%. The MELD score is the only risk stratification tool validated to accurately predict liver-related complications during pregnancy, with a pre-conception MELD score ≥10 indicating a very high-risk of serious liver-related complications. Management necessitates a multidisciplinary approach including a maternal-foetal medicine specialist, hepatologist, neonatologist and anaesthetist. All patients require endoscopy (either before pregnancy or in the second trimester) to assess for the development of varices.
Keywords: Cirrhosis; Portal hypertension; Pregnancy; Varices; Variceal bleeding
Introduction
Pregnancy is an uncommon event in females with liver cirrhosis but represents a complicated and high-risk clinical situation that places both the mother and foetus at risk of serious adverse events. Here we present a case recently managed in our Hepatology unit followed by a review of the literature.
Case Presentation
A 22-year-old primiparous female was admitted to the hepatology ward at 20 weeks gestation
for management of first-presentation decompensated chronic liver disease. This occurred on the
background of long-standing liver cirrhosis with portal hypertension (PH) secondary to inherited
alpha1-antitrypsin (α1AT) deficiency (PiZZ phenotype).
The patient had a past medical history significant for exercise-induced asthma, gastrooesphageal
reflux disease (GORD), childhood obesity, cholelithiasis with previous cholecystitis
(managed conservatively) and visceral hypersensitivity manifesting as recurrent episodes of right
upper quadrant pain. Medications included omeprazole and senna and the patient had documented
allergies to penicillin and non-steroidal anti-inflammatory drugs (NSAIDs). The patient lived
with her partner and was not currently working. She was a life-long non-smoker and consumed
no alcohol. The patient had a strong family history of α1AT deficiency with her mother dying of
emphysema age 50 and a paternal uncle with α1AT deficiency and no known complications.
The patient was diagnosed with α1AT deficiency in 2007 by a paediatric Gastroenterologist
and had compensated cirrhosis with portal hypertension at the time of diagnosis. A gastroscopy in
2007 revealed small (Grade 1) oesophageal varices which required no treatment. The patient was
transitioned to adult care in 2009 and subsequently commenced on a variceal banding programme
for primary prophylaxis in 2010. She was noted to have a long history of stable cirrhosis with no
previous decompensations.
In 2015 the patient noted her menses to be three weeks late and was subsequently found to
be unexpectantly pregnant. She was referred to the high-risk pregnancy clinic at our centre at 16
weeks gestation and a shared-care arrangement with the patient’s Hepatologist was organised. The
patient and her partner were extensively counselled about the risks of pregnancy - which included
liver decompensation, a high-risk of life-threatening maternal haemorrhage and pre-term delivery – however they remained steadfastly of the opinion to continue with
the pregnancy. A second trimester gastroscopy to assess varices was
planned and the patient was informed it was likely non-selective
β-blockers would be commenced to decrease the risk of variceal
haemorrhage. The patient had stable liver function tests (LFTs) and a
normal prothrombin time (PT) of 11 with a baseline Model for Endstage
Liver Disease (MELD) score of 9, but was noted to have a falling
albumin of 22 g/L and chronic thrombocytopaenia (platelets 76 x 109
cells/L) secondary to portal-hypertension-related hypersplenism.
At 20 weeks gestation the patient was admitted with acute
right upper quadrant pain and high-grade fevers secondary to
Streptococcus viridans bacteraemia from a skin infection. This
infection precipitated the patient’s first episode of decompensated cirrhosis with development of jaundice and moderate ascites. The
ascites was managed with introduction of a low-salt diet and diuretics
(spironolactone and furosemide). Intravenous clindamycin was
administered with resolution of symptoms and gradual liver recompensation
(normalisation of bilirubin and resolution of ascites).
The possibility of termination of pregnancy was again discussed
during this admission however the patient was adamant she wished
to continue with the pregnancy.
At 23 weeks gestation the patient was noted to have deteriorating
LFTs with impaired synthetic function (albumin 21, PT 16) and
stable thrombocytopaenia. At 24 weeks gestation the patient was
admitted to the obstetric unit with worsening LFTs, proteinuria
and hypertension and subsequently diagnosed with pre-eclampsia and decompensated cirrhosis. The patient rapidly deteriorated and
emergency caesarean section with delivery of a live male foetus was
performed 24 hours later, at which time large-volume ascites was
noted. The patient was admitted to the intensive care unit (ICU) postoperatively
for management of multiple complications including
acute kidney injury (peak creatinine 280 umol/L) with oligo-anuria
and liver decompensation with development of ascites and jaundice.
Unfortunately, the patient’s son died 48 hours after delivery.
Following discharge from ICU, the patient required prolonged
hospitalisation during which time she was accepted for liver
transplantation assessment. The patient has now received a liver
transplant and remains well.
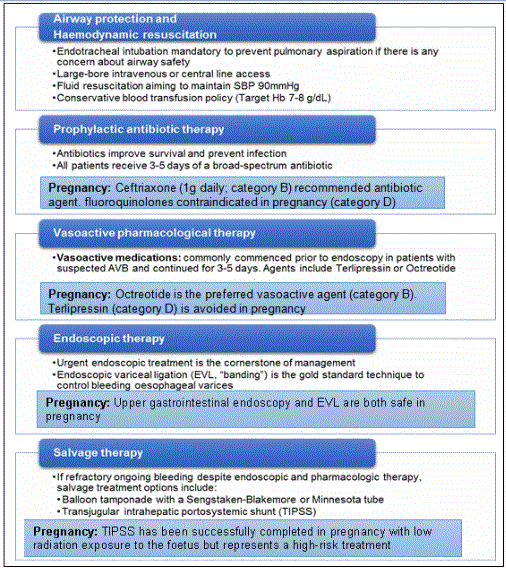
Figure 1
Figure 1
Management algorithm for acute variceal bleeding with pregnancy-specific management options highlighted.
SBP: systolic blood pressure; Hb: Haemoglobin
Discussion
Historically, women with liver cirrhosis were considered infertile
with case reports detailing occasional successful pregnancies. Two
main factors account for pregnancy being an unusual event in
patients with cirrhosis. Firstly, female patients with cirrhosis have
reduced fertility as the metabolic and hormonal derangements
associated with the condition lead to anovulation and amenorrhoea
[1,2]. In addition, it typically takes many years to progress to
advanced liver disease and cirrhosis and thus, the condition is not
commonly encountered in women of child-bearing age [3]. It is clear,
however, that pregnancies in women with cirrhosis are increasing. A
population-based study performed in the USA by Shaheen and Myers
demonstrated an increase in mean nationwide deliveries from 68
annually between 1993 and 1999 to 106 annually between 2000 and
2005 [4]. As treatment of chronic liver disease continues to improve,
it is likely that conception rates and pregnancies in cirrhotic patients
will continue to increase.
Unplanned pregnancy in patients with liver cirrhosis is a highrisk
and complicated clinical scenario with risks to both mother and
foetus. Unplanned and/or unwanted pregnancies represented 38%
of the cohort in one study [5] and expose the mother and foetus to
significant risks. In addition, the safety of planned termination of
pregnancy is not known in cirrhotic patients with evidence limited
to isolated case reports. Thus, although it remains an uncommon
clinical scenario, contraceptive counselling should be provided to all
women of child-bearing age with liver cirrhosis.
The haemodynamic and physiological changes that occur
throughout pregnancy can exacerbate pre-existing PH in women
with cirrhosis. Increases in maternal cardiac output and blood
volume, along with a decrease in systemic vascular resistance result
in a hyperdynamic circulatory state, which increase pressures in the
portal venous system [6]. In women with pre-existing cirrhotic or noncirrhotic
PH 30% - 64% will suffer from liver-related complications
during or after pregnancy, most commonly manifesting as either
variceal haemorrhage or hepatic decompensation [4,6-8]. The largest
case series have shown maternal mortality rates of 1.8% to 7.8% [4,8].
Oesophageal varices
Acute variceal bleeding (AVB) has been reported in 18-32%
of pregnant women with cirrhosis and in up to 50% of those with
known PH [9,10]. In women with known pre-existing varices, up
to 78% will experience gastrointestinal bleeding during pregnancy
with a quoted mortality rate of 18%-50% [2]. These statistics paint a
grim picture but it is important to remember they are based on old
studies. Advancements in the management of AVB have resulted in significant improvements in mortality in non-pregnant patients [11-14] and thus outcomes in pregnant patients are likely to have
also improved. Variceal bleeding most commonly occurs during
the second and third trimesters when maternal blood volume is
maximally expanded [3].
All patients require a screening endoscopy to assess for the
development of varices which typically occurs either before
pregnancy and/or early in the second trimester [15]. Westbrook et al.
demonstrated 50% of screened patients to have varices at this time,
and found a pre-pregnancy platelet count less than 110x109 cells/L
to accurately predict the presence of varices in the second trimester
with a sensitivity of 78% and specificity of 89% [5]. Patients at risk
of variceal bleeding should receive primary or secondary prophylaxis
with either a non-selective beta-blocker or oesophageal variceal
ligation. Beta blockers are generally considered safe in pregnancy;
possible risks include foetal bradycardia, growth retardation and
neonatal hypoglycaemia [6]. Management of AVB is similar to nonpregnant
patients with the exception that octreotide (Category B) is
preferred to terlipressin (category D) as a vasoactive agent (Figure 1).
Hepatic decompensation
Up to 24% of pregnant patients with cirrhosis may experience
liver dysfunction and hepatic decompensation, which can manifest
as jaundice, ascites and/or hepatic encephalopathy [3,16]. Hepatic
decompensation may occur at any stage in pregnancy and risk factors
include variceal bleeding, infection and drugs.
Foetal risks and outcomes
Advanced liver disease is associated with poorer pregnancy
outcomes. Women with liver cirrhosis have a higher incidence of
spontaneous abortion and stillbirth than that of the general population
[3,5], with one retrospective review documenting a live birth rate of
58% [5]. In pregnancies that do result in live births, studies show foetal
complication rates around 49%, with significantly increased risks of
prematurity (39% vs. 10% in the general population), intrauterine
growth restriction (5.3% vs. 2.1%) and perinatal mortality (5.2% vs.
2.1%) [4]. Foetal mortality was high in the setting of maternal hepatic
decompensation (12%) and variceal haemorrhage (11%) [4].
Risk evaluation and counselling in pregnant women with
liver cirrhosis
The complications and consequences of pregnancy in women
with cirrhosis can be variable and unpredictable. Undoubtedly
women with advanced cirrhosis and/or PH are at the greatest
risk of developing significant complications. Westbrook et al. [5]
determined the MELD score was the only risk stratification tool that
accurately predicted complications, with higher MELD scores at the
time of conception correlating with an increased risk of liver-related
complications during pregnancy. Patients with a MELD score of 6
had minimal risk of significant complications. A MELD score of
≥10 prior to conception had an 83% sensitivity and specificity for
predicting a serious liver-related complication during pregnancy or
following delivery [5].
Recommendations for management
Evidence to guide management of pregnancy in patients with
liver cirrhosis is sparse and largely based on case-reports and small
case series. It is clear however that management of these patients
necessitates a multidisciplinary approach including high-risk
obstetric and/or maternal-foetal medicine specialist, hepatologist,
neonatologist and anaesthetist.
Pre-pregnancy management including contraceptive and
pre-conception counselling
In women wishing to become pregnant, extensive preconception
counselling with both an Obstetrician and Hepatologist
is recommended. Patients should be educated about the risks of
pregnancy, which will depend greatly on the aetiology and severity
of the patient’s underlying liver disease. All patients should be risk
stratified using the MELD score and patients with a MELD score
≥10 counselled that they are at particularly high risk of serious
liver-related complications. Pregnancy should be planned when a
patient’s underlying liver disease is stable, with regular hepatology
and obstetric follow-up and close monitoring throughout the entire
ante-partum period. In high-risk patients, alternatives such as liver
transplantation or TIPSS could be considered prior to consideration
of pregnancy.
Antenatal management
Antenatal management requires careful monitoring of the
mother and foetus by a high-risk obstetric/maternal-foetal-medicine
unit and a specialist Hepatologist. Liver function (including liver
synthetic function with serum albumin and INR/PT) should be
checked at least every four weeks and patients should be examined for
signs of decompensation such as ascites, jaundice or encephalopathy.
Full blood count should also be closely monitored as patients with
portal hypertension may develop worsening thrombocytopaenia
or anaemia; anaemia may require treatment to avoid maternal
(cardiac compromise) and foetal (pre-term labour, low birth weight)
complications [6]. Foetal growth also requires careful monitoring.
Pregnancy should proceed to term if the patient and foetus remain
stable. Early termination of pregnancy may be warranted in the
setting of severe liver decompensation or progressive liver failure.
Peripartum management
There are no recommendations as to the preferred mode of
delivery in patients with cirrhosis and PH. Vaginal delivery appears
to be safe and caesarean section is usually reserved for obstetric
indications. In patients with advanced cirrhosis, intra-abdominal
surgery confers a significant risk of liver decompensation and
bleeding risks are high due to coagulopathy, thrombocytopaenia
and the possibility of ectopic varices. The second stage of labour
may be shortened prophylactically to avoid prolonged straining by
the mother, which may increase the risk of variceal haemorrhage.
Variceal bleeding during labour is well described, secondary to
increased intra-abdominal pressure during labour leading to elevated
portal pressures [8,15]. One suggested method to lower risks during
vaginal delivery is to place an epidural early in labour, allow the infant
to descend with uterine contractions alone, followed by assisted
delivery with forceps or vacuum extraction. The third stage of labour
should be managed actively; the risk of post-partum haemorrhage
should be anticipated and vigilantly managed.
Conclusion
Pregnancy in females with liver cirrhosis represents a complicated and high-risk clinical situation that should be managed by a multidisciplinary team including a high-risk obstetric unit and a specialist Hepatologist. If possible, pregnancy should be a planned event that occurs in the setting of stable liver disease and treated varices; this case highlights the dangers of unplanned pregnancies in patients with cirrhosis.
References
- Lloyd CW, Williams RH. Endocrine changes associated with Laennec’s cirrhosis of the liver. Am J Med. 1948; 4: 315-330.
- Russell MA, Craigo SD. Cirrhosis and portal hypertension in pregnancy. Semin Perinatol. 1998; 22: 156-165.
- Tan J, Surti B, Saab S. Pregnancy and cirrhosis. Liver Transpl. 2008; 14: 1081-1091.
- Shaheen AA, Myers RP. The outcomes of pregnancy in patients with cirrhosis: a population-based study. Liver Int. 2010; 30: 275-283.
- Westbrook RH, Yeoman AD, O’Grady JG, Harrison PM, Devlin J, Heneghan MA. Model for end-stage liver disease score predicts outcome in cirrhotic patients during pregnancy. Clin Gastroenterol Hepatol. 2011; 9: 694-699.
- Aggarwal N, Negi N, Aggarwal A, Bodh V, Dhiman RK. Pregnancy with portal hypertension. J Clin Exp Hepatol. 2014; 4: 163-171.
- Sandhu BS, Sanyal AJ. Pregnancy and liver disease. Gastroenterol Clin North Am. 2003; 32: 407-436.
- Rasheed SM, Abdel Monem AM, Abd Ellah AH, Abdel Fattah MS. Prognosis and determinants of pregnancy outcome among patients with post-hepatitis liver cirrhosis. Int J Gynaecol Obstet. 2013; 121: 247-251.
- Britton RC. Pregnancy and esophageal varices. Am J Surg. 1982; 143: 421-425.
- Pajor A, Lehoczky D. Pregnancy in liver cirrhosis. Assessment of maternal and fetal risks in eleven patients and review of the management. Gynecol Obstet Invest. 1994; 38: 45-50.
- Chalasani N, Kahi C, Francois F, et al. Improved patient survival after acute variceal bleeding: a multicenter, cohort study. Am J Gastroenterol. 2003; 98: 653-659.
- Carbonell N, Pauwels A, Serfaty L, Fourdan O, Lévy VG, Poupon R. Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology. 2004; 40: 652-659.
- El-Serag HB, Everhart JE. Improved survival after variceal hemorrhage over an 11-year period in the Department of Veterans Affairs. Am J Gastroenterol. 2000; 95: 3566-3573.
- Vuachet D, Cervoni J-P, Vuitton L, Weil D, Dritsas S, Dussaucy A. Improved survival of cirrhotic patients with variceal bleeding over the decade 2000-2010. Clin Res Hepatol Gastroenterol. 2015; 39: 59-67.
- Esposti SD. Pregnancy in patients with advanced chronic liver disease. Clin Liver Dis. 2014; 4: 62-68.
- Benjaminov FS, Heathcote J. Liver disease in pregnancy. Am J Gastroenterol. 2004; 99: 2479-2488.