Case Report
Treatment of a Myocardial Infiltrating Mediastinal Diffuse Large B-cell Lymphoma: A Case Report
Hassane Abdallah1*, Pier-Alexandre Vasil1, Gabriel Fortin2, Marianne Coutu1 and Sofia Marouan3
1Department of Cardiac Surgery, University of Sherbrooke, Canada
2Department of Internal Medicine, University of Sherbrooke, Canada
3Department of Pathology, University of Sherbrooke, Canada
*Corresponding author: Hassane Abdallah, Department of Cardiac Surgery, University of Sherbrooke,10550 Place de l’Acadie #318, H4N 0B3, Montreal, Quebec, Canada
Published: 17 Aug, 2017
Cite this article as: Abdallah H, Vasil P-A, Fortin G,
Coutu M, Marouan S. Treatment of
a Myocardial Infiltrating Mediastinal
Diffuse Large B-cell Lymphoma: A Case
Report. Ann Clin Case Rep. 2017; 2:
1418.
Abstract
Background: Although primary cardiac lymphoma is rare and challenging to diagnose due to nonspecific symptoms, secondary one is more common, usually from the adjacent lung, breast, esophagus or thymus. However, very few cases describing the treatment of a myocardial infiltrating lymphoma are found in the literature. Case Presentation: We report the case of a 28 year-old immunocompetent woman who presented with rapidly progressing heart failure symptoms with ischemic changes on her electrocardiogram. Different modalities of cardiac imaging showed a large mass compressing and infiltrating the right side of the heart. A biopsy was done and revealed the diagnosis of a B-cell lymphoma. She was treated accordingly with steroids and multi cycles of chemotherapy(R-EPOCH protocol), without any major adverse cardiac event. Conclusion: Although the risk of a myocardial rupture remains possible, we describe a case which demonstrates that a myocardial infiltrating lymphoma can safely be treated with standard chemotherapy regimens.
Keywords: Diffuse large B-cell lymphoma; Myocardial infiltration; Heart rupture
Abbreviations
DLBCL: Diffuse Large B Cell Lymphoma; CT: Computed Tomography; MRI: Magnetic Resonance Imaging; PET: Positron Emission Tomography; R-EPOCH: Rituximab, Etoposide, Prednisone, Vincristine Sulfate, Cyclophosphamide, Hydroxydaunorubicin; STEMI: ST-Elevation Myocardial Infarction; NHL: Non-Hodgkin Lymphoma; PMBL: Primary Mediastinal B-Cell Lymphoma
Introduction
Cardiac Lymphoma is a rare, B-cell non-Hodgkin’s lymphoma. Even though the heart is almost
always involved, it usually is secondary to a systemic malignancy. This means that the lymphoma
may have originated from another part of the body, usually an adjacent organ. In rare instances, it
can be a primary cardiac lymphoma, with only a few cases being reported. Given the rarity of the
primary type, the early diagnosis is often difficult to make. It is generally aggressive and nearly 80%
of the tumor type is diffuse large B-cell type lymphoma (DLBCL) and in some other rare cases,
Burkitt lymphomas.
However, there are many predisposing factors that may contribute to lymphoma formation and
development, but no specific risk factors have been identified for lymphoma involving the heart.
The treatment option depends upon each individual case’s specific circumstances. It varies from
surgical therapy to radio and/or chemotherapy with or without supportive treatment.
Case Presentation
A 28-year-old non-smoker Caucasian woman, mother of three kids, who had no history of any
chronic illness and denied excessive alcohol consumption, presented to the emergency department
complaining of a progressive left shoulder and back pain, palpitations, fatigability, exertional
dyspnea, orthopnea and paroxysmal nocturnal dyspnea. All those symptoms developed over the last few months. She also described a swollen mass on her chest and
another in her back that made it uncomfortable for her to lie on her
back. She had no history of weight loss, but described occasional
night sweats.
On clinical examination, she was fully conscious, alert and
oriented. Her blood pressure was 138/90 millimeter of mercury
(mm Hg), heart rate 118 beats per minute, body temperature 36.4°C
and respiratory rate 20 per minute and oxygen saturation 100% on
room air. An electrocardiogram was immediately done and revealed
an ST-segment elevation in leads V2 through V4 with reciprocal ST
depression in leads III and a VF. Aspirin, Ticagrelor and intravenous
unfractioned heparin were started immediately. Laboratory results
revealed a white blood cell count of 9.1 g/L, hemoglobin of 125 g/L
and platelets of 414 g/L. Troponin I were equivocal (0.090 ng/mL,
normal: < 0.034), lactate deshydrogenase was 418 (elevated). The rest
of the laboratory results were normal. The patient was transferred
to the cardiac catheterization lab for emergency primary coronary
angiography with possible angioplasty.
The coronary angiogram showed no atheromatous lesions of the
coronaries, but it revealed a stiff left anterior descending artery that
did not move in the images because of an anterolateral akinesia.
Echocardiography was performed, in an attempt to understand
the cause of her symptoms and her unusual coronary angiogram
findings. This exam revealed a massive anterior mediastinal mass
that compressed most of the right ventricle and conusarteriosus as
well as a part of the left ventricle. As there was no visible separation
between the pericardium and myocardium in certain views, it was suspected that the tumor infiltrated the myocardium. Moreover, the interior of the left ventricle showed a small mass at the apex, but it was
hard to know if it was a trabeculae or the infiltration by the tumor.
The compression of the heart by the tumor led to an anterolateral,
apical and right ventricular hypokinesia and an acceleration of the
pulmonary artery flow: the maximum and average gradients of the
valve were respectively 42 and 27 mmHg, with the pulmonary artery
systolic pressure estimated at 42+15 mmHg (however difficult to
evaluate due to the presence of a very mild tricuspid regurgitation).
Left ventricular ejection fraction was moderately reduced to 40%-45%.
The inferior vena cava was dilated and not compliant to respiratory
variations. There was a 15 millimeters posterior pericardial effusion.
Cardiac ventriculogram, CT (computed tomography) scan, MRI
(magnetic resonance imagery) (Figure 1) and PET (positron emission
tomography) scan were performed. All those imaging modalities
confirmed the extension of the tumor transmurally, infiltrating the
heart muscle (Figure 2). The mass was extending to the anterior left
hemithorax (Figure 3). A left pleural effusion was also present, as well
as right hilar and paratracheal lymphadenopathies, a left lung nodule,
abdominal lesions and a subcutaneous right hemithorax nodule. The
Ann Arbor staging was determined as IV-X (Figure 4).
The mass measured 14.7 cm (transverse) by 8.7 cm
(anteroposterior) by 17.2 cm (craniocaudal), infiltrating and
compressing the right ventricle and especially the conusarteriosus.
The aorta and pulmonary trunk were displaced by the tumor, but
were still patent (Figure 5).
An excisional biopsy was performed by the general surgery team.
They felt that the most accessible part of the tumor was located at the anterior left hemithorax (Figure 6). The biopsy was performed under local anesthesia in the OR, with a team of anesthesiologist and ENT
surgeon available if there were any complication regarding her airway.
The specimen was sent to our pathology department. It revealed that
the mass was a non-germinal center DLBCL that originated from the
mediastinum. The oncology team installed telemetry as a way to be
warned of any complications and presented the patient to the cardiac
surgery team in case of a cardiac rupture.
Seven days of supportive treatment with corticosteroids was
instituted. The patient had a remarkable improvement in her clinical
symptoms and signs. However, a follow-up echocardiography did not
show any change on the tumor’s effect on the heart.
Chemotherapy was then started with a dose-adjusted R-EPOCH
protocol (Rituximab, Etoposide, Prednisone, Vincristine Sulfate,
Cyclophosphamide, Hydroxydaunorubicin), according to the
National Comprehensive Cancer Network guidelines [1]. Cycles were
scheduled every 3 weeks.
The first three cycles were well tolerated and the cardiovascular
symptoms resolved. Unfortunately, she developed a small bowel
rupture, which was thought to be secondary to the high dosages of
corticosteroids she was receiving. She underwent a successful surgery
with no other complications thereafter.
The PET scan, which was repeated before the fourth cycle showed marked regression in the mass’s size to 5.2 (transverse) x
1.8 (anteroposterior) x 3.1 (craniocaudal) cm. The previous cardiac
infiltration was no longer noticeable.
At latest follow-up, after 5 cycles of chemotherapy, the patient
was back to her normal pre-diagnosis status. All of her cardiovascular
symptoms had resolved, as well as the decline in her general status
that was previously noted. There were no infectious complications
and she had started back to work. Her PET scan showed a complete
remission, with only an anterior mediastinal tissular lesion persisting
that was metabolically inactive.
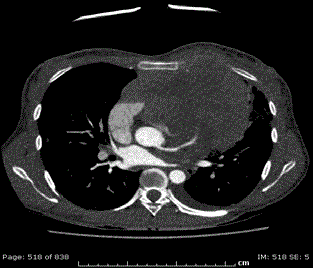
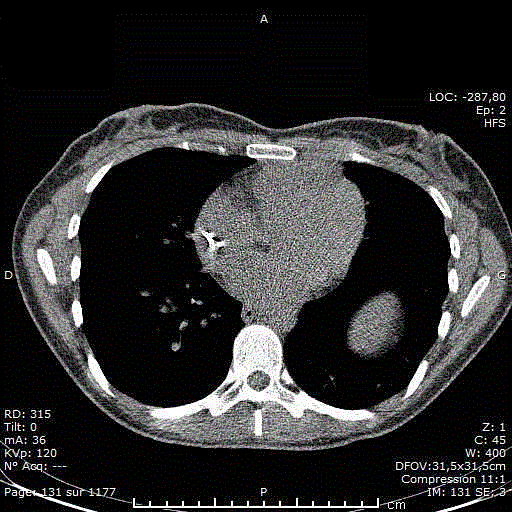
Figure 1
Figure 1
Aortic CECT (contrast-enhanced CT-scan) shows a large
heterogeneous invasive anterior mediastinal mass with pleural, anterior
chest wall and pericardial invasion. Moreover, there is soft tissue infiltration
surrounding the left coronary artery with wall irregularities.
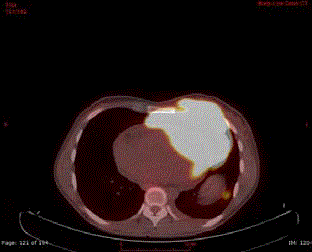
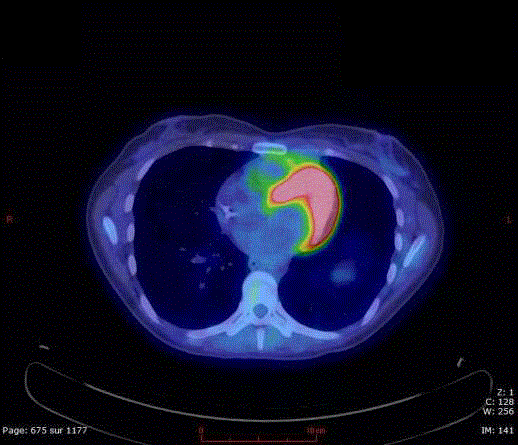
Figure 2
Figure 2
FDG-PET (Fluorodesoxyglucose-positron emission tomography)
scan shows the large hypermetabolic (SUV: 17) anterior mediastinal mass
with evidence of chest wall & pericardial invasion.
Figure 3
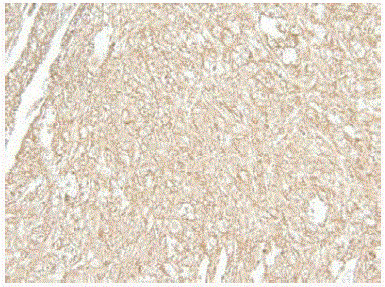
Figure 4
Figure 4
Immunohistochemistry of the left anterior hemithorax specimen
showing a CD20 positive proliferation.
Figure 5
Figure 5
FDG-PET scan (obtained after five chemotherapy cycles) shows
a complete remission with only a metabolically inactive anterior mediastinal
lesion left.
Figure 6
Figure 6
Low-Dose CT-scan (obtained after five chemotherapy cycles)
shows the regression of the anterior mediastinal mass.
Discussion
Symptoms of left heart failure, elevated pulmonary artery
systolic pressure, decreased left ventricular ejection fraction and
STEMI (ST segment elevation myocardial infarction) signs on
the electrocardiogram can all be attributed to the compression
of the heart by the tumor that was proven by different imaging
modalities. Mediastinal tumors are a very rare cause for these clinical
manifestations and would make an interesting discussion. However,
the part that interests us the most for this report is the possibility
of heart rupture caused by melting of the tumor after starting
chemotherapy.
The most common subtype of non-Hodgkin lymphomas (NHL)
is DLBCL [2]. However, primary mediastinal large B cell lymphoma
(PMBL) accounts for only 2.4% of all NHL. These tumors are more
frequent in women and tend to arise in the fourth decade [3]. Their
usual invasive pattern is frequently causing an obstruction of the
airways and/or a superior vena cava syndrome [4]. While small
studies report up to a 48% rate of pericardial effusion associated
with PMBL, [5] documented myocardial infiltration is reported only
anecdotally in the literature.
Some cases of heart rupture secondary to malignant infiltration
have been described, however, they happened before chemotherapy
could be started [6,7]. The reported cases of heart infiltration by
DLBCL treated with chemotherapy showed remission without rupture
[8-10]. We found no reports of rupture secondary to chemotherapy
for infiltrating heart tumor.
Our case seems to follow the few examples that had been reported,
in that the chemotherapy reduced the mediastinal tumor without
causing any major cardiac complications in spite of a transmural
infiltration.
Interestingly, there are case reports that showed the use of cardiac
surgery to remove a symptomatic tumor in the right atrium [11]. In
order to relieve hypoxemia caused by the tumor’s mass effect and
a patent foramen ovale, the intraventricular mass was resected, but
the infiltrating part of the tumor was left in place. Chemotherapy
was started later and the patient went into remission. This seems
to demonstrate that chemotherapy following heart surgery can be
tolerated [12], but in our case, surgery was not indicated because
corticosteroids reduced the tumor volume enough to relieve most of
the symptoms.
Corticosteroids are often used to decrease multiple side effects
of cancer, such as the loss of appetite or the diminished energy. In
addition to these general benefits, steroids are part of virtually all
lymphoma treatment regimens [1]. In fact, it is well known that
lymphocytes are particularly sensible to these molecules, which
induce their apoptosis by different cellular pathways that are not yet completely understood [13]. By this toxicity, they induce a reversal of
most lymphomas, with a concomitant reduction of their mass effect.
However, this effect is only temporary, and chemotherapy must
always follow their initiation [14].
Conclusion
The limited literature shows that myocardial infiltration by a tumor can be treated with chemotherapy, and does not bear a major risk of complication secondary to the tumor melting. Based on the few cases found, we conclude that the untreated tumor puts the patient at greater risk of heart rupture than its treatment by chemotherapy. However, due to the dangerous implications of this complication and the limited data, it should still be anticipated. Cardiac surgery is possible for immediate relief of life-threatening conditions, but corticosteroids followed by chemotherapy are usually enough in the case of PMBL.
Authors Contribution
PAV and GF wrote the initial manuscript. HA and MC reviewed the manuscript. All authors read and approved the final manuscript. The patient gave written informed consent for the publication of the case and the images.
References
- NCCN Clinical Practice Guidelines in Oncology. Non-Hodgkin's Lymphomas. NCCN2016; 3rd edition: BCEL-B 1.
- Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006; 107: 265-276.
- No authors listed. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma.The Non-Hodgkin’s Lymphoma Classification Project. Blood. 1997; 89: 3909-3918.
- Savage KJ. Primary Mediastinal Large B-Cell Lymphoma. The Oncologist. 2006; 11: 488-495.
- Tateishi U, Müller NL,Jonokh T, Onishi Y, Arai Y, Satake M, et al. Primary mediastinal lymphoma: characteristic features of the various histologic subtypes on CT. J Comput Assist Tomogr. 2004; 28: 782-789.
- Armstrong EJ, Bhave P, Wong D, Ursell PC, Kaplan L, Yeghiazarians Y, et al. Left Ventricular Rupture due to HIV-associated T-cell Lymphoma. Tex Heart Instit J. 2010; 37: 457-460.
- Menotti A, Imperadore F, Pelosi G, Disertori M. Heart Rupture at the Right Atrial Level as the First Manifestation of Malignant Lymphoma. Cardiologia. 1996; 41: 65-67.
- Rogowitz E, Babiker HM, Krishnadasan R, Jokerst C, Miller TP, Bookman M. Heart of Lymphoma: Primary Mediastinal Large B-Cell Lymphoma with Endomyocardial Involvement. Case Rep Oncologic Med. 2013; 2013: 814291.
- Okamoto Y, Ueda Y. Successful Treatment of Cardiac Lymphoma. The Japanese Society of Internal Medicine. Intern Med. 2015; 54: 435-436.
- Goedel A, Hoellein A, Rischpler C, Götze K. B-lymphoblastic lymphoma: a heartening diagnosis. Europ Heart J Cardiovasc Imag. 2015; 16: 116.
- Yu PS, Ng CS, Kwok MW, Underwood MJ, Wong RH. Hypoxemia associated with right-side cardiac tumor: right atrial lymphoma with patent foramen ovale. J Thorac Dis. 2016; 8: 527-530.
- Jonavicius K, Salcius K, Meskauskas R, Valeviciene N, Tarutis V, Sirvydis V. Primary cardiac lymphoma: Two Cases and a Review of Literature. J Cardiothorac Surg. 2015; 10: 138.
- Schmidt S, Rainer J, Ploner C, Presul E, Rimi S, Kofler R. Glucocorticoid-induced apoptosis and glucocorticoid resistance: molecular mechanisms and clinical relevance. Cell Death Differ. 2004; 11: 45-55.
- Mckay LI, Cidlowski JA. Corticosteroids in the Treatment of Neoplasms. In: Kufe DW, Pollock RE, Weichselbaum RR, Bast RC, Gansler TS, Holland JF, Frei III E, editors. Holland-Frei Cancer Medicine. 6th edition. Hamilton (ON): BC Decker; 2003.