Case Report
Blue-Finger-Syndrome and Tofacitinib
Popp F1*, Benecke U2, Fischer S3, Kempis J1, Koeger P2 and Mueller RB1
1Department of Rheumatology, Kantonsspital St. Gallen, Switzerland
2Department of Angiology, Kantonsspital St. Gallen, Switzerland
3Department of Anaesthesiology, Luzerner Kantonsspital, Switzerland
*Corresponding author: Florian Popp, Department of Rheumatology, Kantonsspital St. Gallen, Rorschacherstrasse 95, 9007 St. Gallen, Switzerland
Published: 18 Jul, 2017
Cite this article as: Popp F, Benecke U, Fischer S, Kempis
J, Koeger P, Mueller RB. Blue-Finger-
Syndrome and Tofacitinib. Ann Clin
Case Rep. 2017; 2: 1404.
Clinical Image
A 61-year-old male patient with seropositive, ACPA-positive, erosive rheumatoid arthritis (RA) presented with a painful, blue-livid discoloration of his right index finger. Three weeks prior, due to
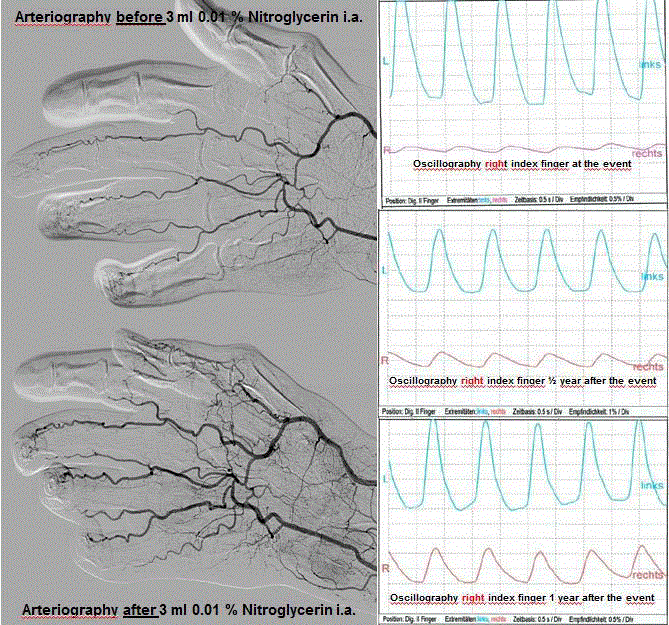
worsening RA-symptoms, RA-therapy was changed from tocilizumab to tofacitinib. Arteriography
showed a significant hypovascularization, oscillography a pathologic pattern. Angiological,
hematological, cardiac and rheumatological workup demonstrated no significant findings but
atherosclerotic changes including the right radial artery. In conclusion the blue-finger-syndrome
can be interpreted as result of atherosclerotic changes, as a potential unexpected adverse event to
tofacitinib or as a combination of both. Tofacitinib was permanently discontinued; iloprost and
enoxaparin were initiated followed by phenprocoumon, which in the further course was replaced by
acetylsalicylic acid. This resulted in rapid improvement of the symptoms. During a one year followup
no additional vascular event was observed.
The blue-finger-syndrome results from blockage, usually thromboembolic or traumatic, of
smaller blood vessels. Consequently, search for thromboembolic sources is required. Further causes
include antiphospholipid-antibody-syndrome, connective-tissue-disease or vasculitis. To our
knowledge, the blue-finger-syndrome has not yet been described under tofacitinib-therapy. In this
patient an increase of LDL-cholesterol has been observed subsequent to initiation of tofacitinib.
Therefore Atorvastatin was started during follow-up. An increase of cholesterol in association with
tofacitinib-therapy [1,2] is reported not to augment the risk of cardiovascular events [1,3,4]. In
general, the cardiovascular safety profile of tofacitinib is similar to other biological disease-modifying
anti-rheumatic drugs [5]. Nonetheless, a drug-associated cause should be discussed (Figure 1).
Figure 1
Figure 1
Ateriography before and after 3 ml 0.01% nitroglycerin i.a. Osicillography right index finger at the year
after the event, Osicillography right index finger ½ year after the event and Osicillography right index finger 1
year after the event.
References
- Jashin J Wu, Bruce E Strober, Peter R Hansen, Ole Ahlehoff, Alexander Egeberg, Abrar A Qureshi, et al. Effects of tofacitinib on cardiovascular risk factors and cardiovascular outcomes based on phase III and long-term extension data in patients with plaque psoriasis. J Am Acad Dermatol. 2016; 75: 897-905.
- Fleischmann R, Kremer J, Cush J, Schulze-Koops H, Connell CA, Bradley JD, et al. Placebo-Controlled Trial of Tofacitinib Monotherapy in Rheumatoid Arthritis. N Engl J Med. 2012; 367: 495-507.
- C Charles-Schoeman, P Wicker, MA Gonzalez-Gay, S P Wood, MG Boy, J Geier, et al. Cardiovascular Safety Findings In Rheumatoid Arthritis Patients Treated With Tofacitinib, A Novel, Oral Janus Kinase Inhibitor. ACR/ARHP Annual Meeting. 2013: 440.
- Christina Charles-Schoeman, Hernan Valdez, Koshika Soma, Lie-Ju Hwang, Ryan DeMasi, Mary Boy, et al. Major Adverse Cardiovascular Events: Risk Factors in Patients with RA Treated with Tofacitinib. ACR/ARHP Annual Meeting 2016; 3024.
- Wollenhaupt J, Silverfield J, Lee EB, Curtis JR, Wood SP, Soma K, et al. Safety and Efficacy of Tofacitinib, an Oral Janus Kinase Inhibitor, for the Treatment of Rheumatoid Arthritis in Open-label, Longterm Extension Studies. J Rheumatol. 2014; 41: 837-852.