Case Report
Contralateral Crossrecurrence of a Malignant Glioma: A Case With Long-Term Survival
S Ferraresi1*, E Brognaro1, G Pavanato2 and MR Ballotta3
1Department of Neurosurgery, Ospedale S.Maria della Misericordia, Italy
2Department of Radiotherapy, Ospedale S.Maria della Misericordia, Italy
3Department of Pathology, Ospedale S.Maria della Misericordia, Italy
*Corresponding author: Valentino MA, Division of Cardiology, Department of Internal Medicine, Thomas Jefferson University Hospital, Philadelphia, PA, USA
Published: 03 Jul, 2017
Cite this article as: Valentino MA, Saxena S, Vira A, Tecce
M. Propionibacterium acnes Infection
of a Mitral Annuloplasty Ring. Ann Clin
Case Rep. 2017; 2: 1343.
Abstract
In surgically treated glioblastomas a recurrence of the primitive tumour with subsequent infiltration of the surrounding parenchyma is the commonest cause of death.
In the vast majority of the cases it occurswithin or nearby thetumour site. However, in a few patients
the recurrence is locatedfar from the primary tumor site and radiological heralding signs may even
not be visible.
This paper illustrates the clinical and radiological patterns of a 56 year old woman with a right
occipital glioblastoma and a late contralateral cross recurrence in the left frontal lobe.
After a complete surgical removal, the patient experienced an high quality 6 year-survival period.
The tumour was expressing the promoter to methylation but was wild type for IDH mutation. It was
treated with radiotherapy plus Temozolamide.
Lately she developed a very unusual contralateral cross-recurrence with these cond tumour
genetically similar to the original one (MGMT promoter and IDH wild type) but rapidly and
relentlessly fatal.
As a peculiar event we have an MRI, incourse of a routine control done 4 months before the second
hospitalization, which shows no sign of recurrence.
This casts a further light on the speed and modality of growth of an high grade glioma.
Introduction
Despite advances in several oncological fields, the prognosis of patients affected by glioblastoma is still poor and the median survival remains very short (15 months).
The general features for glioblastomas quote a 3-5% survival rate at three years and 2-3% at 5
years [1,2]. In the patients receiving the Stupp protocol we find a 16% rate at 3 years and 10% at 5
years [3,4].
Some patients survive even more. The features that allow to live longer are not completely
understood but the progress achieved in neurosurgical techniques, focal radiotherapy and
chemotherapy with temozolomide have certainly helped. They are referred to as long-term survivors
and their number seems to be strictly dependent on the molecular pattern of the glioblastoma,
namely the MGMT promoter methylation, the IDH status and the combination of the two, as it has
been recently stated in the paper by Molenaar et al. [5].
In some cases the cause of death reportedly has no correlation with the original glioblastoma
nor with complications or sequelae of its treatment [6,1]. However, the majority of patients die from
one or more relapses of the original glioblastoma. Most occur in the original surgical siteor adjacent
to it, but 5%-20% of recurrences are found to emerge distant in the ipsilateral hemisphere or even
in the contralateral one [7]. Very often, these cases lack of signal abnormalities in T2 MRI and no
leptomeningeal involvement is visible, neither in the brain parenchyma adjacent to the original
glioblastoma nor in the contralateral area of relapse.
A deeper understanding of such cases may be helpful to gain in sights into the entire development
of the glioblastoma both concerning the bulk and the surrounding parenchyma.
Case Presentation
A 56-year-old women was admitted, at the end of November
2006, because of generalized seizures. She showed apathia, mild
disorientation, sensorial disphasia and had been complaining about
visual disturbances for a few days before admission. At neurological
examination a right homonimous lateral hemianopsia was present.
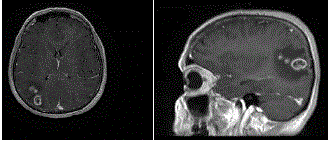
The MRI revealed a right parieto-occipital contrast enhancing tumor
(Figure 1). Spectroscopy also suggested the presence of an high grade
glioma.
The patient was operated on and the tumour completely removed.
The postoperative follow-up was uneventful. Histopathological
examination revealed a WHO grade IV glioma. The proliferation index
was 22% and the MGMT-promoter methylated. The IDH status was
wild type, as it usually happens in the newly diagnosed glioblastomas
[8]. The patient was treated according to Stupp's protocol [4] with
conformal radiotherapy and adjuvant chemotherapy. The total dose
delivered with linear accelerator was 60 Gy in 30 fractions of 2 Gy 5 days a week during a 6 week period.
Daily temozolomide, 75 mg per square meter of body-surface
area, was administered during radiotherapy. After a 4-week break, six
monthly cycles of adjuvant temozolomide, at daily dose of 200 mg for
square meter, 5 days per week, were added.
The patient was regularly examined and remained in good health.
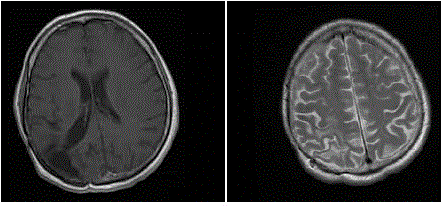
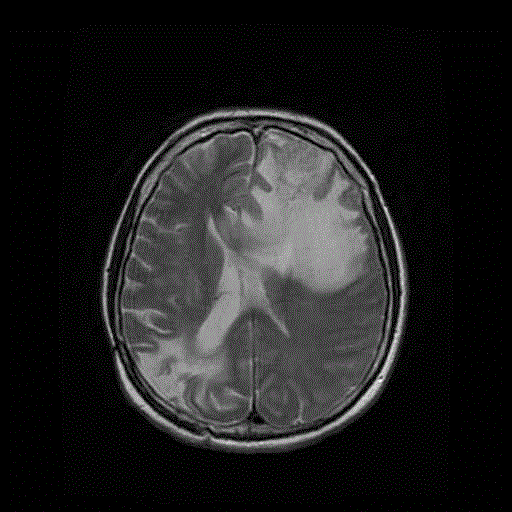
An MRI done at the end of September 2011 (5 years after surgery) still
showed no signs of relapse (Figure 2 and 3). Then the patient was readmitted
in our department at the beginning of February 2012. She
showed disorientation and uncertain walking during the last week.
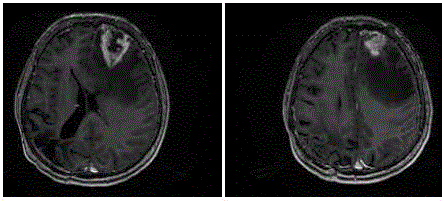
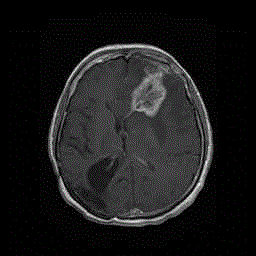
A CT scan displayeda space occupying lesion surrounded by edema
in the left frontal region. MRI (Figure 4) and spectroscopy again
oriented for a high grade glioma. T2 MRI contrast enhanced scan still
did not show any connection between the left frontal lesion and the
original right parietal-occipital glioblastoma (Figure 5). The patient
underwent a new surgery in February 2012 and the postoperative
follow-up was good.
The histopathological examination confirmed a WHO grade
IV glioma , Ki-67-positive cells at 17% rate, the MGMT-promoter
methylated and IDH wild-type [9,10]. The molecular pattern was so
the same of the original tumour.
Being the new lesion in the contralateral hemisphere, the
patient was enrolled once more for Stupp'sprotocol with conformal
radiotherapy and adjuvant temozolomide. Finally, she died at the end
of 2012, approximately one year after the second surgery, due to a
local recurrence (Figure 6) and subsequent diffusion of the disease.
Figure 1
Figure 2
Figure 3
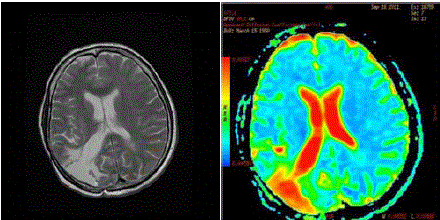
Figure 3
(left) 2011 - basal T2 MRI. No signs of microinfiltration along neural
pathways 4 months before the recurrence. (right) SPECT done 4 months
before the recurrence. No signs ofabnormalities in the brain parenchyma
herald a cross contralateral diffusion. The colour scale has been arbitrarily
inverted: red indicates fluid (CSF) and poroencephaly in the right occipital
lobe.
Figure 4
Figure 5
Figure 5
MRI February 2012 - T2 scan without contrast enhancement –
Still novisible connection between the site of the newly diagnosed left frontal
tumor and the primary right parietooccipital glioblastoma removed in 2006.
Figure 6
Figure 6
December 2012 - Contrast enhanced MRI – Recurrence of the
left frontal tumour removed in February 2012. Note that the site of the first
surgery (right occipital) remained disease-free.
Discussion
The long-term survival of patients diagnosed with glioblastoma
multiforme is a rare but possible event [11]. Their number has slightly
increased in the last years compared to the past [1,2,12]. The clinical
and molecular features that allow some patients to live longer are
under debate. To date, the more relevant factor (present in 74%
of patients evaluated) contributing to a prolonged survival is the
methylation of the MGMT promoter, particularly in those patients
receiving focal radiotherapy and adjuvant temozolomide [10].
In particular, this turns to be true in young patients with a good
Karnofsky personal score (KPS) at the time of the diagnosis.
Socioeconomic, environmental and occupational factors had no
relevance at all [13]. In this case a new tumor, with a second stem
cell giving origin to a second glioblastoma, cannot be excluded.
Also, a malignancy associated with the radiotherapy could also be
the case but the simpler, and probably most obvious solution, is a
relapse caused by one of the cancer stem cells settled in the whole
parenchyma from the site of the primary glioblastoma since its onset
[14]. The identical molecular patterns of the two tumours, even after
years, strongly favour this hypothesis. Recently, researchers have
documented that a hierarchical model with the cancer stem cells at
the top of the pyramid [15,16,17] exist in the glioblastoma and in
other tumor types. It is highly probable that they remain quiescent
in the brain until when, for some reasons, reactivate. In histological
brain sections of mice transplanted with glioblastoma cells, a gradient
of invasive tumor cells is documented. This means that outside the
macroscopical bulk of the glioblastoma, there may be a decreasing
number of micro infiltrating CSCs which settle quiescent in the
parenchymal niche. Indirect evidence of this is drawn from the early
unsuccessful attempts made by neurosurgeons to remove an entire
right emisphere affected by a glioblastoma. Their efforts failed due to
relapse in the contralateral hemisphere.
In our patient, however, the absolute absence of any sign of
recurrence in the original tumour bed until only 4 months before,
confirmed by an MRI with contrast enhancement and a spectroscopy,
sounds extremely peculiar and has motivated the case illustration.
It seems unbelievable, in fact, that no signs of recurrence have
been observed in the contrast enhanced MRI along the white
interconnecting fibers.
Conclusion
This case emphasizes once morethe importance of the surgical act and that, probably, the recurrences originate in two different ways. The first and more common modality, derives from active Cancer Stem Cells (CSCs) of the bulk tumor not completely removed at time of first surgery. The second, and less frequent, probably stems from quiescent CSCs settled in the parenchymal niche. The identification of membrane antigens or receptors identifying these stray cells located along theso called glial fields or “Gliarasen” of the German neuropathologists, could be of utmost importance for the development of new therapeutic agents against the glioblastoma.
References
- Hottinger AF, Yoon H, DeAngelis LM, Abrey LE. Neurological outcome of longtermglioblastoma survivors. J Neurooncol. 2009; 95: 301-305.
- Scott JN, Rewcastle NB, Brasher PM, Fulton D, Hagen NA, MacKinnon JA, et al. Long-term glioblastoma multiforme survivors: apopulation-based study. Can J Neurol Sci. 1998; 25: 197-201.
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effect of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009; 10: 459-466.
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fischer B, Taphoorn MJ, et al. Radiotherapyplus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352: 987-996.
- Molenaar RJ, Verbaan D, Lamba S, Zanon C, Jeuken JW, Boots-Sprenger SH, et al. The combination of IDH mutations and MGMT methylation status predicts survival in glioblastoma better than either IDH1 or MGMT alone. Neuro Oncol. 2014; 16: 1263-1273.
- Bähr O, Herrlinger U, Weller M, Steinbach JP. Very late relapses in glioblastomalong-term survivors. J Neurol. 2009; 256: 1756-1758.
- Wick W, Stupp R, Beule AC, Bromberg J, Wick A, Ernemann U, et al. A novel tool toanalyze MRI recurrence patterns in glioblastoma. Neuro Oncol. 2008; 10: 1019-1024.
- Banan R, Hartmann C. The new WHO 2016 classification of brain tumours – what neurosurgeons need to know Acta Neurochir. 2017; 159: 403-418.
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006; 444: 756-760.
- Millward CP, Brodbelt AR, Haylock B, Zacharia R, Baborie A, Crooks D, et al. The impact of MGMT methylation and IDH-1 mutation on long-term outcome for glioblastoma treated with chemoradiotherapy. Acta Neurochir. 2016; 158: 1943-1953.
- Fatoo A, Nanaszko MJ, Allen BB, Mok CL, Bukanova EN, Beyene R, et al. Understanding the role of tumor stem cells in glioblastoma multiforme:a review article. J Neurooncol. 2011; 103: 397-408.
- Steinbach JP, Blaicher H-P, Herrlinger U, Wick W, Nagele T, Meyermann R, et al. Surviving glioblastoma for morethan 5 years: the patient's prospective. Neurology. 2006; 66: 239-242.
- Krex D, Klink B, Hartmann C, von Deimling A, Pietsch T, Simon M, et al. Long term survival withglioblastoma multiforme. Brain. 2007; 130: 2596-606.
- Brognaro E. A theory and a model to understand glioblastoma development both inthe bulk and in the microinfiltrated brain parenchyma. Neurochem Res. 2011; 36: 2145-2154.
- Beier D, Hau P, Proescholdt M, Lohmeier A, Wishhusen J, Oefner PJ, et al. CD133+ and CD133- glioblastoma-derived cancer stemcells show differential growth characteristics and molecular profiles. Cancer Res. 2007; 67: 4010-4015.
- Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004; 64: 7011-7021.
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004; 432: 396-401.