Case Report
Choroid Plexus Papilloma Causing CSF Shunt Ascites: A Rare Presentation
Deepak Sachan*
Department of Pediatrics, Postgraduate Institute of Medical Education and Research, Dr. Ram Manohar Lohia
Hospital, New Delhi, India
*Corresponding author: Deepak Sachan, Department of Pediatrics, Dr. Ram Manohar Lohia Hospital, New Delhi, India
Published: 13 Jun, 2017
Cite this article as: Sachan D. Choroid Plexus Papilloma
Causing CSF Shunt Ascites: A Rare
Presentation. Ann Clin Case Rep. 2017;
2: 1376.
Abstract
Choroid Plexus Papillomas (CPPs) are congenital intracranial tumors of neuro-ectodermal origin. Choroid plexus neoplasms constitute about 0.5% of all intracranial neoplasms.Majority are found in lateral ventricles. Most of these neoplasms are benign papillomas, while one-fifth are malignant carcinomas. The present communication describes a rare case of a choroid plexus papilloma leading to CSF ascites following Ventriculoperitoneal (VP) shunt.
Case Presentation
A 5 year old boy presented to us with complaints of progressively increasing abdominal
distension from past 6 months and respiratory distress for 2 days. There was no history of jaundice
or bleeding manifestations. Patient was a known case of hydrocephalus for which medium pressure
VP shunt (chabra shunt) was placed at the age of 3 years. On examination child was having
massive ascitis with positive fluid thrill sign. There was no hepato-spenomegaly and other signs of
hepatocellular failure. Neurologically the child was conscious and oriented and there were no signs
of shunt dysfunction. Shunt bulb was palpable and soon gets refilled after compressing the bulb.
Paracentesis showed clear transudate fluid with no evidence of infection (WBC= 5 cells/mm3, all
lymphocytes, sugar = 72 mg/dl and protein = 24 mg/dl). Ascitic fluid culture was sterile and was
negative for Acid fast bacilli. In addition, cytology was negative for malignant cell. Liver and renal
function test were essentially normal (serum bilirubin = 0.6 mg/dl, SGOT = 36 U/I, SGPT = 11 U/I,
serum albumin = 3.8 gm/dl, urea = 27 mg/dl, creatine = 0.8 mg/dl). Echocardiography revealed a
normal functioning heart. The patient tested negative for HIV. Abdominal Ultrasound and CT scan
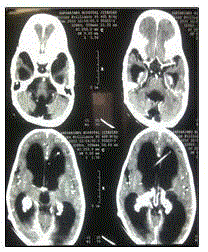
showed no abnormality in relation to abdominal viscera or peritoneum. CECT head showed gross
communicating hydrocephalus with choroid plexus papilloma (Figure 1) in bilateral lateral ventricle which was later confirmed by histopathological examination of postoperative specimen.
Despite diuretic treatment and peritoneal tapping the ascites re-accumulated. Based on
impression of CSF ascites the lower end of the shunt tip was exteriorized and was maintained as
ventricular drain so as to relieve intracranial and intra-abdominal pressures, which continued
to drain 1200-1500 ml of CSF daily and ascites resolved within 2 weeks postoperative. Surgical
resection of choroid plexus papilloma was done on both sides of lateral ventricles and shunt was
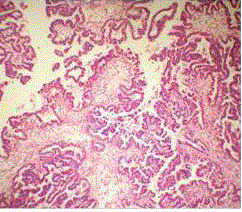
removed. The postoperative period was uneventful. Sections from both right and left tumour
tissue show multiple fragments composed of delicate fibrovascular fronds lined by single layer of
monomorphic cuboidal cells with basal round nucleus. No necrosis, mitosis or pleomorphism seen.
S-100 immunostain shows positivity in tumour cells. Above histological features are suggestive of
choroid plexus papilloma (Figure 2).
Figure 1
Figure 2
Figure 2
Photomicrograph (H&E) showing fibrovascular fronds lined by
single layer of monomorphic cuboidal cells with basal round nucleus of
choroid plexus papilloma.
Discussion
Ascites has been defined as accumulation of excess fluid within the peritoneal cavity [1]. The commonest cause of ascites is cirrhosis of the liver, closely followed by other serious hepatic diseases [2]. In children, hepatic, renal and cardiac diseases are the most common causes. CSF ascites is a rare complication of Ventriculoperitoneal (VP) shunts. VP shunts are usually placed for obstructive
or progressive hydrocephalus. Occlusion of the shunt tube and infection are frequently observed as
V-P shunt complications. Overproduction of the CSF will be the likely possibility once the shunt
infection had been ruled out. Early detection of shunt ascitis (noninfective) which is an uncommon
occurrence and its aetiology will be helpful for better management.
Different intervals (2 months- 13 years) between shunt placement and symptomatic ascites have been reported [3-6]. Our case develops ascitis after two and half
years of shunt placement. Several etiologic factors had been discussed
in literature, but it is the imbalance between peritoneal absorption
capacity and amount of CSF Production is the major cause. By
this definition, patients with excessive amount of CSF production
like choroid plexus papilloma are at risk to developing CSF ascites
following VP shunt [3,7,8]. On the other hand, patients with high
CSF protein due to chronic infection (tuberculous meningitis)
[9] or brain tumors –especially optic glioma [4,8,10] may have difficulties in CSF absorption through peritoneum. Under such
circumstances, inflammation has been associated with an increase in
leukocytes, impairment of lymphatic flow, and a subsequent increase
in intraperitoneal protein concentration due to impaired protein
absorption causing ascitis.
Peritoneal inflammation due to repeated shunt revisions [5] or
non-specific inflammatory response to shunt material [9], play role in
the other side and decrease absorptive ability of peritoneum. Also in
brain tumors, especially in astrocytoma and glioblastoma, increased
vascular permeability can cause microvascular extravasation of
plasma into the peritoneal cavity and cause ascites [11-14]. A large
series of twenty-eight patients with cerebrospinal ascites have been
reported [5]. Their ages ranged from 10 days to 53 years, but most patients were children, especially infants. Common etiological
factors responsible were congenital hydrocephalus, obstructive
hydrocephalus, choroid plexus papilloma, craniopharyngioma and
posterior fossa tumour. In our patient choroid plexus papilloma was
found to be the cause of CSF ascites. This is thought to be due to
imbalance between excess production and its absorption.
Treatment for cerebrospinal ascites is revision of the V-P shunt to
ventricular-atrial shunt but in choroid plexus papilloma revision will
only relieve ascitis with associated risk of congestive heart failure and
bacteraemia. Surgical resection of the papilloma is the definitive cure.
References
- Podolsky DK, Isselbacher K. Major complications of cirrhosis. Fauci AS, Braunwald E, Isselbacher KJ, (eds). In: Harrisons Principles of Internal Medicine. McGraw-Hill, New York. 1998; 1710-1716.
- Glickman RW, Isselbacher KJ. Abdominal swelling and cirrhosis. Fauci AS, Braunwald E, Isselbacher KJ, (eds), In: Harrisons Principles of Internal Medi-cine. New York, McGraw-Hill. 1998: 255-257.
- Pawar SJ, Sharma RR, Mahapatra AK, Lad SD, Musa MM. Choroid plexus papilloma of the posterior third ventricle during infancy and childhood: Report of two cases with management morbidities. Neurol India. 2003; 51: 379-382.
- Gil Z, Beni-Adani L, Siomin V, Nagar H, Dvir R, Con-stantini S. Ascites following ventriculoperitoneal shunting in children with chiasmatic-hypothalamic glioma. Childs Nerv Syst. 2001; 17: 395-398.
- Yukinaka M, Nomura M, Mitani T, Kondo Y, Tabata T, Nakaya Y, et al. Cerebrospinal ascites developed 3 years after ventriculoperitoneal shunting in a hydroce-phalic patient. Intern Med. 1998; 37: 638-641.
- Longstreth GF, Buckwalter NR. Sterile cerebrospinal fluid ascites and chronic peritonitis. N Engl J Med. 2001; 345: 297-298.
- Fujimoto Y, Matsushita H, Plese JP, Marino R Jr. Hy-drocephalus due to diffuse villous hyperplasia of the choroid plexus: Case report and review of the literature. Pediatr Neurosurg. 2004; 40: 32-36.
- Fujimura M, Onuma T, Kameyama M, Motohashi O, Kon H, Yamamoto K, et al. Hydrocephalus due to cere-brospinal fluid overproduction by bilateral choroid plexus papillomas. Childs Nerv Syst. 2004; 20: 485-488.
- Yaqoob N, Abbasi SM, Hussain L. Cerebrospinal fluid ascites. J Coll Physicians Surg Pak. 2003; 13: 289-290.
- West GA, Berger MS, Geyer JR. Childhood optic path-way tumors associated with ascites following ventricu-loperitoneal shunt placement. Pediatr Neurosurg. 1994; 21: 254-259.
- Takano S, Yoshii Y, Kondo S, Suzuki H, Maruno T, Shirai S, et al. Concentration of vascular endothelial growth factor in the serum and tumor tissue of brain tumor patients. Cancer Res. 1996; 56: 2185–2190.
- Kanayama H, Yano S, Kim SJ, Ozawa S, Ellis LM, Fidler IJ . Expression of vascular endothelial growth factor by human renal cancer cells enhances angio-genesis of primary tumors and production of ascites but not metastasis to the lungs in nude mice. Clin Exp Me-tastasis. 1999; 17: 831-840.
- Strugar JG, Criscuolo GR, Rothbart D, Harrington WN. Vascular endothelial growth/permeability factor ex-pression in human glioma specimens: correlation with vasogenic brain edema and tumor-associated cysts. J Neurosurg 1995; 83: 682-689.
- Verheul HM, Hoekman K, Jorna AS, Smit EF, Pinedo HM. Targeting vascular endothelial growth factor blockade: ascites and pleural effusion formation. On-cologist. 2000; 1: 45-50.