Case Report
Large Biopsy-Related Renal Allograft Pseudoaneurym: Evident Sonographically but Indistinct Angiographically
Waleska M Pabon-Ramos*
Department of Vascular and Interventional Radiology, Duke University Medical Center, USA
*Corresponding author: Waleska M. Pabon-Ramos, Department of Vascular and Interventional Radiology, Duke University Medical Center, Erwin Road, Duke North, Box 3808 Durham, NC 27710, North Carolina, USA
Published: 09 Jun, 2017
Cite this article as: Pabon-Ramos WM. Large
Biopsy-Related Renal Allograft
Pseudoaneurym: Evident
Sonographically but Indistinct
Angiographically. Ann Clin Case Rep.
2017; 2: 1371.
Abstract
Percutaneous renal biopsies may be complicated by iatrogenic pseudoaneurysms. These are frequently diagnosed sonographically, and treated angiographically. This case report presents a renal transplant patient whose renal function declined after percutaneous renal biopsy. A large (5.5 cm) biopsy-related renal transplant pseudoaneurysm was readily identified on ultrasound, but was not visualized on selective renal transplant arteriography. Subsequent super selective renal transplant arteriography revealed the pseudoaneurysm, which was successfully embolized. One month after embolization, the patient’s renal function nearly normalized.
Keywords: Renal transplant pseudoaneurysm; Renal transplant complication; Renal transplant iatrogenic injury; Renal transplant biopsy; Renal embolization
Case Presentation
A 62 year-old male with end-stage renal disease status post living unrelated renal transplant to the right iliac fossa presented with a slowly increasing serum creatinine. Baseline serum creatinine level was 1.1 mg/dL three days after transplantation, but increased to 1.7 mg/dL thirteen days after transplantation. That day nephrology performed an ultrasound-guided percutaneous renal allograft biopsy. Two passes were made with a Max Core® spring-loaded 14 G by 16 cm core biopsy needle (Bard®, Tempe, AZ), and two samples were obtained. Pathology findings were nonspecific. The mild renal dysfunction was attributed to tacrolimus toxicity since trough levels had been borderline high. However, despite tacrolimus dose adjustments and eventual discontinuation, serum creatinine level continued to gradually increase. Twenty-five days after renal transplantation (12 days after renal allograft biopsy) serum creatinine had risen to 2.5 mg/dL. That day nephrology performed a second ultrasound-guided percutaneous renal allograft biopsy. Five passes were made with a Max Core® 14 G by 16 cm core biopsy needle, and two samples were obtained. Pathology was suspicious for acute rejection. Subsequent to the second biopsy, serum creatinine level rapidly increased to 6.4 mg/dL, which prompted a renal allograft ultrasound.
Imaging Findings
Thirty-three days after renal transplantation (8 days after the second renal allograft biopsy)
a renal allograft ultrasound was performed. Grayscale images revealed a 5.5 cm x 3.7 cm x 5 cm
complex cystic structure (Figure 1a) at the renal allograft hilum. On color Doppler, the structure demonstrated red and blue turbulent flow (Figure 1b), suggesting a pseudoaneurysm. On spectral Doppler, the neck demonstrated a biphasic waveform (Figure 1c), confirming the presence of a pseudoaneurysm. The component of the pseudoaneurysm containing flow measured 2 cm in
diameter. Transcatheter embolization was requested given the rapid post-biopsy rise in creatinine,
overall diameter of the pseudoaneurym, and diameter of the patent component.
The same day of the ultrasound, digital subtraction arteriography of the right external iliac
artery and transplanted renal artery was performed in oblique projections. A total of 96 mL of
Visipaque 320 mgI/mL (GE Healthcare, Mississauga, ON) was used. These arteriograms revealed
a 1 cm pseudoaneurysm in the lower pole of the renal allograft (Figure 2). A lesion corresponding
to the 5.5 cm hilar pseudoaneurysm shown by ultrasound was not identified. The findings were
discussed with the transplant surgery and nephrology teams, and embolization of the 1 cm lower
pole pseudoaneurysm was deferred since the risk of post-embolization renal infarction and possible
consequent decrease in renal function outweighed the benefit of embolizing a small pseudoaneurym.
The next day, another renal allograft ultrasound re-demonstrated the 5.5 cm hilar
pseudoaneurysm. It was stable in overall diameter, but there had been some interval thrombus regression paralleled by an increase in the diameter of the patent
component to 3 cm. The transplant surgery and nephrology teams
requested another renal transplant arteriogram with transcatheter
embolization. Obtaining a CT angiogram for lesion delineation and
endovascular treatment planning was considered but deferred given
the patient’s acute renal dysfunction.
Thirty-four days after renal transplantation, a repeat renal
transplant arteriogram was performed, and showed interval increase
in diameter of the previously identified lower pole pseudoaneurysm
to 1.4 cm. Then, super selective arteriography of the lower pole
segmental artery feeding the pseudoaneurysm revealed a larger
pseudoaneurysm component just medial to and communicating with the previously identified small lower pole pseudoaneurysm (Figure 3).
Management
The decision was made to embolize the pseudoaneurysm. A
microcatheter (Renegade, Boston Scientific, Natick, MA) was
advanced into the interlobar branch directly feeding the bilobed
pseudoaneurysm, and the branch was embolized with one 3/2 mm
Tornado microcoil (Cook, Bloomington, IN). Completion super
selective arteriography demonstrated complete occlusion of the
pseudoaneurysm’s feeding vessel; renal infarction was not identified
(Figure 4). A total of 50 mL of Visipaque 320 mgI/mL was used.
Follow-up
The patient was discharged two days after embolization. His
serum creatinine level decreased gradually after the procedure, and
reached a new baseline of 1.6 mg/dL one month after the procedure.
He did not require hemodialysis after embolization (Table 1).
Figure 1
Figure 1
Renal allograft ultrasound (Siemens ACUSON Sequoia 512 ultrasound system, 6C2-S probe) (a) Gray scale image in longitudinal orientation
demonstrates a complex cystic structure at the hilum (white arrowhead) measuring 5.6 cm x 3.7 cm x 5 cm. (b) Color Doppler image in longitudinal orientation at
the same level as in (a) demonstrates red and blue turbulent flow within the cystic structure, also referred to as the “ying-yang” sign (white arrowhead). (c) Spectral
Doppler demonstrates a biphasic waveform at the pseudoaneurysm neck, also referred to as “to-and-fro” flow.
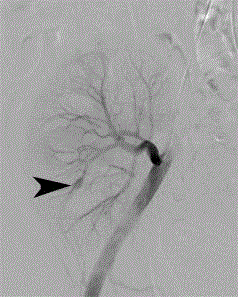
Figure 2
Figure 2
Selective renal transplant arteriogram (Siemens Artis fluoroscopy
unit) through a left femoral arterial approach demonstrates a 1 cm intra renal
pseudoaneurysm (black arrowhead) at the lower pole of the renal allograft.
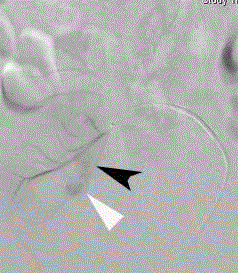
Figure 3
Figure 3
Super selective renal transplant arteriogram through a right
femoral arterial approach reveals a larger pseudoaneurysm component
(black arrowhead) medial to and communicating with the previously identified
1 cm lower pole pseudoaneurysm (white arrowhead).
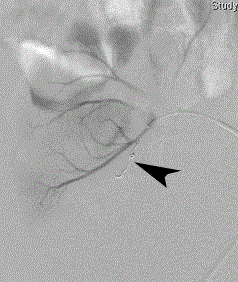
Figure 4
Figure 4
Post-embolization super selective renal transplant arteriogram
demonstrates complete occlusion of the pseudoaneurysm’s feeding arterial
branch with one 3/2 mm Tornado microcoil.
Discussion
Etiology and demographics
Biopsy-related iatrogenic vascular injuries are rare. They occur
in 0.3% of renal transplant recipients, and in 1-18% of renal allograft
biopsies [1-3]. Arteriovenous fistulae are the most common vascular
injury, followed by pseudoaneurysms, occurring in 0.2% and 0.08%
of renal transplant recipients, respectively [1]. There is no gender or
age predilection, but more biopsy-related iatrogenic vascular injuries
occur in adults because more adult patients receive renal transplants.
Pseudoaneurysms result from isolated laceration of the arterial
wall [4]. Typically, extra renal pseudoaneurysms develop at the arterial anastomosis secondary to surgical technique or perigraft
infection, while intra renal pseudoaneurysms develop at the renal
poles secondary to percutaneous biopsies [5,6].
Clinical and imaging findings
Most biopsy-related iatrogenic renal pseudoaneurysmsare
asymptomatic, are found incidentally on surveillance imaging, and
resolve spontaneously [7]. When symptomatic, they may present
with massive or persistent hematuria, obstructive uropathy from clot
retention, or an elevation in serum creatinine level [6,8,9].
Initial evaluation of suspected iatrogenic renal pseudoaneurysms
is performed with ultrasonography. Contrast enhanced CT scans
may also be used, but they are usually avoided since administration
of intravenous iodinated contrast may exacerbate underlying renal
dysfunction. Arteriography is performed if treatment is warranted.
Although on gray-scale ultrasound pseudoaneurysms appear
as simple or complex renal cysts, their color and spectral Doppler
ultrasound characteristics are pathognomonic. Color Doppler
ultrasound shows turbulent flow in the central lumen - typically
depicted by alternating jets of forward and reverse color - described
as the “ying-yang” sign. Spectral waveforms at the pseudoaneurysm
neck demonstrate a classic biphasic “to-and-fro” pattern [5,7].
Detection of intra renal vascular complications by ultrasound has
a reported sensitivity and specificity of 77% and 100%, respectively
[2]. Angiographically, pseudoaneurysms commonly have a lobular
appearance; less commonly, the injured vessel demonstrates contour
abnormality, or an abnormal caliber change.
In our case, ultrasonography was unequivocal in revealing a
pseudoaneurysm, but localized the lesion to the renal allograft hilum.
After the initial iliac and selective renal transplant arteriograms failed
to reveal a pseudoaneurysm at the renal hilum, super selective renal
arteriograms of the upper and lower poles should have been obtained
because these are the areas typically biopsied. This would have revealed
the larger component of the lower pole bilobed pseudoaneurysm.
Alternatively, carbon dioxide renal arteriography could have
been performed. Carbon dioxide is not nephrotoxic, and has been
described to be more sensitive in the identification of biopsy-related
renal arteriovenous fistulas when compared to iodinated contrast
[10]. The increased sensitivity was attributed to the lower viscosity
of carbon dioxide, especially when compared to that of Visipaque,
which is the iodinated contrast medium usually selected for patients
with underlying renal dysfunction [10]. Carbon dioxide arteriography
may also have a higher sensitivity in the detection of biopsy-related
pseudoaneurysms.
Treatment and prognosis
The treatment of choice for biopsy-related iatrogenic renal
pseudoaneurysms, especially in the setting of renal allografts, is
transcatheter super selective embolization. Embolization should
be performed if the lesion is symptomatic, or if asymptomatic but
progressively enlarging or larger than 2 cm in diameter. Super
selective embolization has a technical success rate of 95%-100%. The
clinical sign or symptom that prompts the procedure resolves in 88%-
100% of patients [9,11].
If the lesion is not amenable to super selective embolization, super
selective embolization has failed, or the procedure is not available, the
indicated treatment is partial or complete nephrectomy. However,
compared to these surgical alternatives, super selective embolization
preserves the most renal function.
After super selective embolization, 10%-32% of patients
experience progressive deterioration of renal function [9,11]. Whether
all of these patients experience renal impairment as a consequence of
the procedure remains uncertain. Nonetheless, cases in which super
selective embolization causes significant renal parenchyma infarction
may result in decreased renal function [9,11,12].
Table 1
Table 1
Summary table of the key characteristics and imaging findings of biopsy-related renal transplant pseudoaneurysms [1,4,5,9,11].
Conclusion
If a biopsy-related renal transplant pseudoaneurysm is identified on ultrasound, but not on selective renal arteriography, super selective upper and lower pole renal arteriograms should be performed because the pseudoaneurysm is most likely to be found in these areas. The treatment of choice for this lesion is transcatheter super selective embolization.
References
- Arya S, Coleman DM, Osborne NH, Michael Englesbe, Eva Rzucidlo, Peter K Henke, et al. Outcomes of endovascular interventions for salvage of renal transplant allografts. J Vasc Surg. 2013; 57: 1621-1627.
- Grenier N, Claudon M, Trillaud H, Douws C, Levantal O. Noninvasive radiology of vascular complications in renal transplantation. Eur Radiol. 1997; 7: 385-391.
- Johnson SP, Berry RS. Interventional radiological management of the complications of renal transplantation. Semin Intervent Radiol. 2001; 18: 47-58.
- Friedewald SM, Molmenti EP, Friedewald JJ, Dejong MR, Hamper UM. Vascular and nonvascular complications of renal transplants: sonographic evaluation and correlation with other imaging modalities, surgery, and pathology. J Clin Ultrasound. 2005; 33: 127-139.
- Dodd GD, Tublin ME, Shah A, Zajko A. Imaging of vascular complications associated with renal transplants. Am J Roentgenol. 1991; 157: 449-459.
- Jin KB, Hwang EA, Han SY, Park SB, Kim HC, Kim YH, et al. Delayed presentation of arteriovenous fistula and pseudoaneurysms in a renal transplant patient 10 years after percutaneous allograft biopsy. Transplant P. 2008; 40: 2444-2445.
- Akbar SA, Jafri SZ, Amendola MA, Madrazo BL, Salem R, Bis KG. Complications of Renal Transplantation. Radiographics. 2005; 25: 1335-1356.
- Sharma AK, Sunil S, Rowlands P, Bakran A. Pseudoaneurysm with severe hematuria in renal allograft after renal biopsy treated by percutaneous embolization. Nephrol Dial Transpl. 2002; 17: 934-935.
- Perini S, Gordon RL, LaBerge JM, Kerlan RK, Wilson MW, Feng S, et al. Transcatheter embolization of biopsy-related vascular injury in the transplant kidney: immediate and long-term outcome. J Vasc Interv Radiol. 1998; 9: 1011-1019.
- Cheng PM, Van Allan RJ. Superior sensitivity of angiographic detection of arteriovenous fistula after biopsy in a renal allograft with CO2 compared with iodinated contrast medium. J Vasc Interv Radiol. 2006; 17: 1963-1966.
- Maleux G, Messiaen T, Stockx L, Vanrenterghem Y, Wilms G. Transcatheter embolization of biopsy-related vascular injuries in renal allografts. Acta Radiol. 2003; 44: 13-17.
- Surlan M, Popovic P. The role of interventional radiology in management of patients with end-stage renal disease. Eur J Radiol. 2003; 46: 96-114.