Case Report
Acute Cerebrovascular Accidents Secondary to Internal Carotid Artery Thrombosis in Severe Ovarian Hyperstimulation Syndrome (OHSS)
Chin-Man Wang1*, Yi-Chou Wang2, Yin-Chou Lin1, Yu-Wei Hu1 and Ji-Yih Chen3
1Department of Physical Medicine and Rehabilitation, Chang Gung Memorial Hospital and Chang Gung University, Taiwan
2Department of Neurosurgery, Chang Gung Memorial Hospital and Chang Gung University, Taiwan
3Department of Medicine, Chang Gung Memorial Hospital and Chang Gung University, Taiwan
*Corresponding author: Chin-Man Wang, Department of Physical Medicine and Rehabilitation, Chang Gung Memorial Hospital, No 5, Fu-Hsin Street, Kuei Shan, Tao-Yuan, 333, Taiwan
Published: 31 May, 2017
Cite this article as: Wang C-M, Wang Y-C, Lin Y-C, Hu
Y-W, Chen J-Y. Acute Cerebrovascular
Accidents Secondary to Internal Carotid
Artery Thrombosis in Severe Ovarian
Hyperstimulation Syndrome (OHSS).
Ann Clin Case Rep. 2017; 2: 1362.
Abstract
Severe Ovarian Hyperstimulation Syndrome (OHSS) is an uncommon iatrogenic complication, and
complicated cerebral infarctions is exceedingly rare.
We present a case of 39-year-old young woman with left cerebral infarctions due to OHSS in
first trimester of pregnancy after ovulation induction therapy. At beginning, she was treated with
paracentesis conservatively at same clinic of district hospital. Later on, she was transferred to our
medical center due to neurological deterioration from large left hemispheric infarctions with pending
uncal herniation, and the craniotomy and lobectomy were performed in our medical center. The
pregnancy was terminated at 12 weeks gestation for management of OHSS complicated with cerebral
infarctions. Due to left hemispheric infarctions, she had neurological deficits with the functional
impairments of right hemiplegia and global aphasia. After 6 weeks of intensive rehabilitation, she
was able to ambulate with assistance but could speak only a few words at discharged.
Knowledge of OHSS facilitates early detection and prevents catastrophic complications, and
intensive hospitalized treatment is indicated for serious OHSS. According, neurological deficits
with OHSS should be considered as criteria for hospitalization.
Keywords: Ovarian hyperstimulation syndrome; Thromboembolic stroke
Introduction
The Ovarian Hyperstimulation Syndrome (OHSS) is an iatrogenic complication of Ovulation- Induction Therapy (OIT) for Assisted Reproductive Technology (ART) which is rare but lifethreaten in severe form. The prevalence of the severe OHSS ranges from 0.1% to 5% among patients undergoing OIT [1-3]. The pathophysiology remains uncertain. It consists of ovarian enlargement accompanied by overproduction of ovarian hormones and a host of other ovarian vasoactive substances including cytokines, angiotensin, and vascular endothelial growth factor, which alone or in concert produces a state of increased capillary hyperpermeability [4]. Clinical manifestations are varied, such as ovarian enlargement, abdominal distension, ascites, pleural effusion, and electrolyte disturbances. Severe OHSS can lead to multiple organs failure and thromboembolic events [5]. Thromboembolism is rare but serious. A systematic review of literatures included 68 reported OHSS cases, in which 65.7% of thrombi occurred in the venous system, while 34.3% in the arterial system [6]. Thromboembolism in the brain is the most feared complication of OHSS [5,7], despite treatment the neurological deficits with functional disability or even the unfortunate death in productive young women might be happen. Here, a case of OHSS complicated multiple left cerebral infarctions during her first trimester of pregnancy after OIT is documented.
Case Presentation
A 39-year-old, right handed, pregnant woman was admitted to our hospital with the chief
complaint of sudden loss of consciousness and right-side weakness. She had undergone OIT with
follitropin-beta injection of 200–250 IU for 9 days at a district hospital, 3 weeks before admission.
Abdominal distension appeared 1 week after she finished the course of follitropin-beta. OHSS
was suspected. Sonography revealed bilateral enlarged ovaries (right 9.7 cm x 9.1 cm; left 7.5 cm x 4.4 cm), and ascites. Ascites fluid (2,200 ml) was removed by
paracentesis. Two days prior admission to our hospital, she had
a short episode of syncope and aphasia for hours after regaining
consciousness. She was sent to a district hospital and discharged
home on the next day, with a total recovery and negative findings
on blood tests. At home, on the day of admission to our hospital,
she had another episode of consciousness loss associated with right
side weakness. After intravenous hydration with 2,000 ml normal
saline at same district hospital, she was transferred to our hospital
due to persistent impaired consciousness. On admission, her blood
pressure was 126/74 mmHg, with a heartbeat of 102 beats/min
and body temperature of 36.8°C. On examination, she was drowsy
with severe right side weakness and global aphasia. OHSS with
ischemic stroke was diagnosed. Laboratory tests demonstrated that
the serum white blood cell counts (18.8 x 103/μL; normal, 3.5-11 x
103), c-reactive protein (130.62 mg/dL; normal, <5.0), fibrinogen
(594 mg/dL; normal, 198-380) and D-dimer (2,006.12 ng/mL; cutoff
at 500) elevated, while serum albumin (2.1 g/dL; normal, 3.5-5.5),
hemoglobin (11.8 mg/dL; normal, 12-16) and hematocrit (35.0%;
normal 36-46) were decreased. Human chronic gonadotropin was
389.5 mIU/mL (not pregnant, <5). Prothrombin time, activated
partial thromboplastin time, homocysteine, antithrombin III, C3, C4,
protein C, and protein S were within normal limits. Anticardiolipin
antibody, antiphospholipid antibody, antinuclear antibody were
negative. Transthoracic echography showed no cardiac abnormalities.
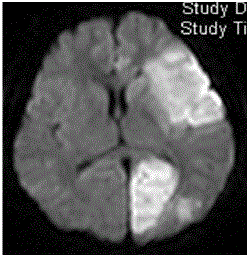
Magnetic resonance imaging showed ischemic lesions on T2-
weighted and diffusion weighted imaging in the left frontotemporal
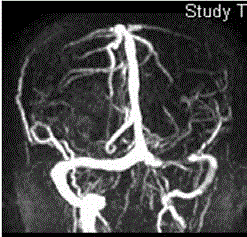
lobe, insular cortex, and left occipital lobe (Figure 1). Magnetic
resonance angiography showed total occlusion of the left internal carotid artery (Figure 2). The patient had no history of hypertension, diabetes mellitus, hyperlipidemia, cardiac abnormalities, illicit drug
abuse, and neither family history of thromboembolic disease nor OHSS.
The patient was admitted to the Intensive Care Unit (ICU). Over
the next few days, she received conservative and supportive treatment
with IV fluids, albumin and mannitol infusions, with close monitoring
of her physical and neurological conditions. Her pregnancy was
decided to be terminated after consultation by her medical team
with her family on her fourth day in ICU. Unfortunately, she
became more lethargic and her right pupil became dilated, without
any light reflex on the next day. New neurological deterioration was
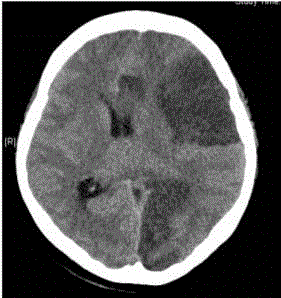
noted, and emergency brain computerized tomography revealed
large infarctions in the left hemisphere causing a rightward midline,
uncal herniation, and effacement of the cortical sulci (Figure 3).
She underwent a craniotomy and left temporal lobectomy due to
neurological deterioration and increased intracranial pressure.
Next day, transvaginal echography showed a small gestational sac
(maximal diameter, 0.92 cm) without a fetal heartbeat; left ovary
measured 10.4 cm x 12.1 cm, right ovary measured 7.4 cm x 6.2
cm, and a small amount of ascites fluid noted in the cul-de-sac.
The pregnancy was terminated at 12 weeks of gestation. She was
transferred to rehabilitation department 3 weeks after brain operation,
with right hemiplegia and global aphasia. After 6 weeks of intensive
rehabilitation, she could ambulate with assistance for 10 meters but
say only a few words. C-reactive protein, fibrinogen, D-dimer, and
albumin returned to normal, and she was discharged with aphasia
and poor communication ability. One year after the left hemispheric
infarctions, she still had right hemiplegia but can walk independently.
Her speech had some improving with few short phrases.
Figure 1
Figure 1
Magnetic resonance imaging of the brain showed multifocal
high-intensity lesions on diffusion-weighted imaging, involving the left
frontotemporal lobe, insular cortex, and left occipital lobe, suggestive of
recent infarcts.
Figure 2
Figure 3
Figure 3
Computerized tomography revealed large infarctions in the left
cerebral hemisphere with a midline shift, and subfalcine and uncal herniation.
Discussion
There are few reports of cerebral infarction complicating OHSS in
the medical literature [5,7-10]. In our patient, the thrombotic events
occurred in left carotid artery territory, resulting in multiple cerebral
infarctions is rare. This clinical picture differs from the previously
reported cases by its extent of the infarctions and the fulminating
process. Ovulation induction with daily administration of 200-250
units of Follitropin-beta injection is not a careful treatment regimen
though not exceeding the maximal dose suggested by company.
The cause of OHSS associated thromboembolic disease is not
clearly understood, but it appears to be related to high estrogen
concentrations, low plasma volume, and hemoconcentration [11]. In
our patient, a low serum albumin level at admission suggested fluid shift to the third space, with reduced intravascular volume. Increased
levels of fibrinogen and D-dimer were observed on admission and
normalized by the follow-up examination with supportive treatment.
The presence of progressive, multi-territorial infarctions with
alterations of hemostatic factors and the absence of a cardioembolic
source, suggested a hypercoagulable state, which could have been
responsible for her cerebral infarctions. Her initial hematocrit and
hemoglobin were not increased, which could have been partly due
to the effect of hemodilution treatment for OHSS at the infertility
clinic. However, high estrogen level is an established hypercoagulable
state. Antithrombin III, protein C, protein S, anticardiolipin IgG, and
antiphospholipid antibody were all within normal limits, excluding
other hypercoagulable states.
The treatment of OHSS is aimed at supportive conservative
measures to prevent and counter hemoconcentration. Identification
of mild to moderate cases of OHSS is essential to prevent the rare,
severe complications. Severe OHSS requires immediate therapy under
close monitoring. Careful clinical examination to detect the presence
of secondary complications is essential for the prevention of severe
OHSS [12,13]. Hematocrit is a valuable parameter for evaluation of
the severity of OHSS. One criteria for hospitalization is hematocrit
rises to 45% form previous literature [6]. According to this case report,
we suggest that other clinical neurological symptoms and signs must
be considered and combined for hospitalization required. Heparin
should be given when the thromboembolic risk is markedly increased,
such as with hyperestrogenemia, immobilization, compression
of the pelvic vessels by enlarged ovaries or ascites, and pregnancy
coagulation anomalies. Prevention of signs and symptoms by the use
of mobilization and anti-thrombosis stockings is insufficient because
the etiology of thrombosis is of a systemic nature [6]. Furthermore,
early treatment with intra-arterial Recombinant Tissue Plasminogen
Activator (rt-PA) in OHSS complicated by thromboembolic stroke
was reported in a case report, with successful results [7]. This
treatment needs a careful evaluation for indicated patients with OHSS
complicated thromboembolic stroke to avoid complication, also the
time window for aggressive rt-PA treatment is not yet determined.
Due to delay diagnosis and large multiple infarctions, the intraarterial
rt-PA was not given in our case. Abdominal paracentesis may
be needed for symptomatic relief of tense ascites. It is also indicated in
the setting of oliguria, increasing creatinine or decreasing creatinine
clearance, and hemoconcentration refractory to medical therapy.
Surgical intervention for OHSS should be avoided unless hemorrhage
of an ovarian cyst, cystic rupture, or torsion of the ovary is suspected.
Termination of pregnancy may be necessary in a few rare cases,
especially in those with severe OHSS complications refractory to
medical therapy, in an effort to decrease the serum human chronic
gonadotropin level [4]. As with our patient, severe OHSS with
thrombotic events that worsen after conservative treatment under
close monitoring make termination of pregnancy inevitable due to
without a fetal heartbeat.
Prophylaxis heparin is debatable since there are no randomized
studies proving its efficacy in preventing thromboembolic
complications during severe OHSS. Although, heparin was given,
thromboembolism was reported in some patients. Besides, the timing
for administration is undetermined. Some favor maintaining heparin
therapy for at least 4 weeks and even during the whole first trimester of
pregnancy, others suggest before the appearance of OHSS symptoms
[6]. However, this should be applied to patients with known preexisting
thrombophilic factors. In this case, she was treated without heparin at outside district clinics and due to no known history of thrombophilic factors. Evaluation of antithrombin III, protein C,
protein S, and mutation of Factor V, II and MTHFR genes should
be performed for patients with a personal and family history of
thromboembolic episodes or previous OHSS [6]. The prevention
and treatment of OHSS should be individualized or standardized
which are still controversial [12,14]. However, early identification of
OHSS and thromboembolic risk factors is helpful for prevention and
management of thromboembolic events occurring in critical OHSS.
Conclusion
Since ARTs are used much more common for infertility in recent days. Early diagnosis and hospitalization for those rare serious OHSS to prevent further catastrophic or life-threatening complications including the thromboembolic stroke is necessary.
References
- Delvigne A, Rozenberg S. Epidemiology and prevention of ovarian hyperstimulation syndrome (OHSS): a review. Hum Reprod Update. 2002; 8: 559-577.
- Golan A, Ron-el R, Herman A, Soffer Y, Weinraub Z, Caspi E. Ovarian hyperstimulation syndrome: an update review. Obstet Gynecol Surv. 1989; 44: 430-440.
- Serour GI, Aboulghar M, Mansour R, Sattar MA, Amin Y, Aboulghar H. Complications of medically assisted conception in 3,500 cycles. Fertil Steril. 1998; 70: 638-642.
- Budev MM, Arroliga AC, Falcone T. Ovarian hyperstimulation syndrome. Crit Care Med. 2005; 33: S301-S306.
- Bartkova A, Sanak D, Dostal J, Herzig R, Otruba P, Vlachova I, et al. Acute ischaemic stroke in pregnancy: a severe complication of ovarian hyperstimulation syndrome. Neurol Sci. 2008; 29: 463-466.
- Delvigne A, Rozenberg S. Review of clinical course and treatment of ovarian hyperstimulation syndrome (OHSS). Hum Reprod Update. 2003; 9: 77-96.
- Elford K, Leader A, Wee R, Stys PK. Stroke in ovarian hyperstimulation syndrome in early pregnancy treated with intra-arterial rt-PA. Neurology. 2002; 59: 1270-1272.
- Demirol A, Guven S, Gurgan T. Aphasia: an early uncommon complication of ovarian stimulation without ovarian hyperstimulation syndrome. Reprod Biomed Online. 2007; 14: 29-31.
- Yang S, Li R, Chen XN, Fu Y, Yi M, Ma CH, et al. Acute Cerebral Thrombosis Following Ovarian Hyperstimulation Syndrome: A Case Report. Chin Med J (Engl). 2015; 128: 3383-3384.
- Girolami A, Scandellari R, Tezza F, Paternoster D, Girolami B. Arterial thrombosis in young women after ovarian stimulation: case report and review of the literature. J Thromb Thrombolysis. 2007; 24: 169-174.
- Stewart JA, Hamilton PJ, Murdoch AP. Thromboembolic disease associated with ovarian stimulation and assisted conception techniques. Hum Reprod. 1997; 12: 2167-2173.
- Nelson SM. Prevention and management of ovarian hyperstimulation syndrome. Thromb Res. 2017; 15: S61-S64.
- Corbett S, Shmorgun D, Claman P. The prevention of ovarian hyperstimulation syndrome. J Obstet Gynaecol Can. 2014; 36: 1024-1033.
- Fiedler K, Ezcurra D. Predicting and preventing ovarian hyperstimulation syndrome (OHSS): the need for individualized not standardized treatment. Reprod Biol Endocrinol. 2012; 10: 32.