Case Report
A Case of New Manifestation of Leprosy Six Months after Immigration to Germany
Sogkas Georgios1, Pott Carl-Christoph1, Schacht Vivien2, Sedlacek Ludwig3, Schmidt Reinhold Ernst1 and Stoll Matthias1*
1Department of Immunology and Rheumatology, Hannover Medical University, Germany
2Department of Dermatology, Hannover Medical University, Germany
3Department of Medical Microbiology, Hannover Medical University, Germany
*Corresponding author: Matthias Stoll, Department of Immunology and Rheumatology, Clinic for Immunology and Rheumatology, Hannover Medical University, Carl- Neuberg-Strasse 1, 30625, Hannover, Germany
Published: 12 May, 2017
Cite this article as: Georgios S, Carl-Christoph P, Vivien S,
Ludwig S, Ernst SR, Matthias S. A Case
of New Manifestation of Leprosy Six
Months after Immigration to Germany.
Ann Clin Case Rep. 2017; 2: 1358.
Abstract
Leprosy is a chronic infectious disease mainly prevalent in subtropical and tropical areas, caused by Mycobacterium leprae. It typically affects the skin and the peripheral nervous system. Leprosy presents in distinct clinical forms, depending on the immune response, elicited to combat Mycobacterium leprae. We report the case of a previously asymptomatic 28-year-old male from Sudan, who was admitted with since five months appearing progressive bilateral leg and hand edemas. Skin biopsy revealed a multibacillary mycobacterial granulomatous inflammation and molecular analysis for Mycobacterium leprae was positive, confirming the diagnosis of leprosy. This case emphasizes that leprosy can also appear in people from endemic areas immigrating asymptomatic to Europe, several months after immigration.
Keywords: Leprosy; Mycobacteria; Mycobacterium leprae; Refugee; Migrants; Europe; Germany
Introduction
Leprosy (or Hansen’s disease) is a chronic infectious disease mainly of the skin and nerves,
caused by Mycobacterium leprae [1]. Mycobacterium leprae is an obligatory intracellular pathogen,
whose exact mode of transmission remains unclear. However, a recent study by Araujo “et al.” [2] strongly supports an aerosol route of transmissionand infection [2].
Leprosy is an important public health problem in the subtropics and tropics. In Germany and
the rest of Europe, leprosy is an extremely rare infection, mainly imported by immigrants from
endemic areas [3]. Growing migration flows from endemic regions to Europe and other countries, where leprosy is considered eradicated, is expected to increase its incidence. Further, due to the
rarity of leprosy in Europe, it can be difficult for physicians to consider leprosy as a differential
diagnosis, which may delay diagnosis. Delayed diagnosis may have debilitating consequences,
including blindness and permanent nerve damage [4].
We report the case of a previously asymptomatic 28-year-old male from Sudan with progressive
peripheral edema, which was diagnosed with leprosy.
Case Presentation
A 28-year-old male-a refugee from south Sudan – who had been living in Germany for 9 months
was referred to our clinic by his general practitioner with bilateral leg and hand edema of unclear
etiology.
On admission the patient complained of progressive edema in his both legs and hands as well
as of his eyelids. Moreover, he had numbness in the affected areas of legs and hands. He reported
repeated burn injuries in his hands, which according to him happened all recently, due to the absence
of thermal sensitivity in his peripheral extremities. He also complained of progressive weakness in
his legs. The aforementioned problems started gradually during the last three months. Since a couple
of months he had fever; a maximum auricular temperature of 38.6ºC was reported. Weight loss and
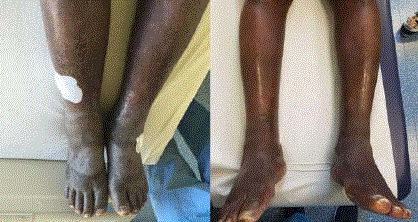
night sweat were denied. The clinical examination revealed symmetric extremely dense edemas of
lower legs with diffuse hyperkeratosis, a few palpable nodular changes, some of which were also
viewable and a few hypo pigmented macules (Figure 1). Similar - though less pronounced - edemas
were observed in the dorsum of both hands and in the fingers. A few nodular changes were palpable
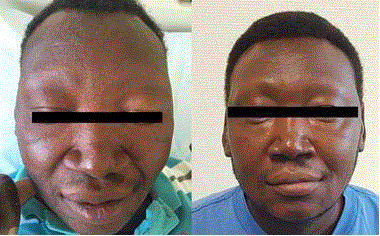
in the lateral sides of both elbows of the patient. In the face there was bilateral periorbital edema of soft consistency with loss of eyelashes and eyebrows (Figure 2). The
neurological examination revealed loss of touch and pin sensation in
the affected edematous areas.
On admission the C-Reactive Protein (CRP) was elevated (41.9 mg/l, normal range < 5 mg/l). The full blood count revealed a slight hypochromic anemia. Other routine laboratory tests were normal,
except for a slight elevation of gamma-glutamyl-transferase (gGT,
126 U/l, normal range < 55 U/l) and Alkaline Phosphatase (ALP,
190, normal range 40-129 U/l). An HIV-test was negative. We also
performed an interferon-gamma release assay (IGRA), which was
positive. However, all specimens submitted for microbiological
testing remained culturally and PCR negative for Mycobacterium
tuberculosis. In addition, serum analysis for leishmaniosis and
syphilis as well as blood analysis for microfilariosis were negative.
A conventional chest X-ray and an abdominal sonography
revealed no pathological findings. An electroneurography revealed
substantially reduced motor and sensory potentials in the lower legs
(nervus peronaeus, nervus tibialis, nervus suralis) as wells as the
forearm and the hands (nervus medianus), indicating severe axonal
polyneuropathy.
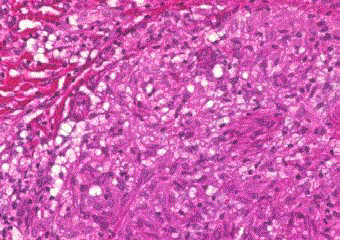
The histological examination of a skin-biopsy from the left
leg revealed the formation of multiple granulomata and areas with
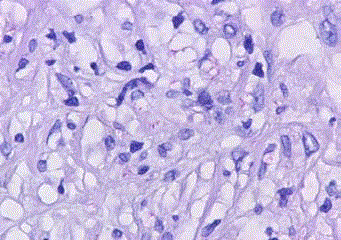
lymphocytic infiltration (Figure 3). The acid-fast stain revealed
lots of mycobacteria diffuse in the dermis (Figure 4). Polymerase
Chain Reactions (PCR) were employed to specify the mycobacterial
species. A PCR analysis of the bacterial 16S rRNA gene, followed by
sequencing identified Mycobacterium leprae, whereas the molecular
analysis for Mycobacterium tuberculosis was negative. Evaluation of
the mycobacterial load in skin-biopsy stained with Ziehl–Neelsen
stain, revealed approximately 80 bacilli per high powered field, matching a bacterial index of 4+ [5]. A Fite-Faraco-stain, which is more sensitive at identifying Mycobacterium leprae, was not
performed, as it is unavailable in our Pathology lab.
Taken the above described clinical findings (numerous macular
and nodal skin lesions with partially symmetric distribution,
pronounced symmetrical edema of lower legs, severe polyneuropathy)
and the results of the histological examination we made the diagnosis
of multibacillary form of leprosy.
Based on our diagnosis, we started a multidrug therapy with
daily administration of 100 mg dapsone, 50 mg clofazimine as well
as a supervised monthly administration of 600 mg rifampicin and
300 mg clofazimine. Due to the progressive weakness of the lower
legs, which started gradually in the last month, and the findings of
the electroneurography, we set the diagnosis of a lepra reactionmediated
neuronal injury, so that we started an anti-inflammatory
therapy with 50 mg prednisolone, whose dose was slowly tapered.
This led to an alleviation of the numbness and could reverse the
weakness of the lower legs, strongly suggesting the coexistence of
an inflammatory lepra reaction. In the follow-up, two months later,
the edemas of the lower legs (Figure 1), hands and face (Figure 2) had substantially regressed. The macular and nodular changes of
skin disappeared and CRP was normal. However, loss of sensitivity
could not be reversed.The above mentioned therapy with dapsone,
clofazimine and rifampicin should be continued for ten more months
(1-year regimen).
Figure 1
Figure 1
Symmetrical edema of lower legs on presentation (left) and a
month after introduction of therapy (right).
Figure 2
Figure 2
Bilateral periorbital edema with loss of eyelashes and eyebrows
(middle photo), compared to the ID-photo, which was taken 9 months ago
(left) and the follow-up photo, which was taken 1 month after therapyintroduction
(right).
Figure 3
Figure 3
Skin-biopsy: hematoxylin and eosin stain. Dermis with formation of
a granuloma with macrophages surrounded by lymphocytes (evident at the
barrier between granuloma and connective tissue at the lower left corner of
this figure) (40x magnification).
Figure 2
Figure 4
Follow-up trans-esophageal echocardiogram.
(A-B) 2-chamber view of the mitral valve in systole (A) and diastole (B) with a
large vegetation attached to the annuloplasty ring and posterior mitral valve
leaflet.
(C) 3D TEE image of the mitral valve in systole with a large vegetation
attached to the mitral annuloplasty ring.
Discussion
Leprosy (or Hansen’s disease) is a chronic infection endemic
in the subtropics and tropics, causing a broad range of clinical manifestations [1]. The causative agent is Mycobacterium leprae.
Leprosy typically presents with cutaneous manifestations.
Involvement of the peripheral nerves with sensory loss is also
common. Apart from direct pathogenicity of the chronic infectious
process, leprosy may be complicated with immunologic reactions,
which drive the mounting of acute inflammatory manifestations.
These can appear before, during or after starting an antimycobacterial
treatment (reverse reaction) and may lead to permanent damage if
not treated with immunomodulatory and anti-inflammatory agents.
The therapy of leprosy depends on its form [1]. Classification of
leprosy is based on clinical, immunological and histopathological
criteria [6]. The phenotype of borderline leprosy, sharing features
of both the two classical extreme clinical phenotypes of tuberculoid
and lepromatous leprosy, was sub-classified in the classification of
Ridley and Jopling, which is based on clinical and histopathological
criteria (Tuberculoid Tuberculoid-TT, Borderline Tuberculoid-
BT, Borderline Borderline-BB, Borderline Lepromatous-BL,
Lepromatous Lepromatous-LL) [7]. In case of our patient, despite
the clinical presentation with a bilateral symmetrical involvement
pattern, including symmetrical edema of the lower legs and feet,
which is a typical feature of LL leprosy, the histological findings
matched BL leprosy. This discrepancy between the histological
and clinical phenotype, suggests the existence of overlapping
phenotypes of leprosy, including features of neighboring forms. In
1982 the World Health Organization (WHO) proposed a primarily
bacteriological classification, based on the number of acid-fast bacilli
in the dermis [6]. Bacilli-counts are expressed as bacteriologic index
on a logarithmic scale. This classification distinguishes between a
paucibacillary form, matching TT- and BT-form, and a multibacillary
form, matching the BB-, BL- an LL-form of the Ridley-Jopling
classification. Because of the absence of required infrastructure
in areas with the highest disease prevalence for a classification
dependent on bacilli-counts, the WHO suggested in 1988 a clinical
classification based on counting of skin lesions; patients with 5 or less
lesion should be treated as paucibacillary leprosy, whereas patients
with more than 5 lesion should be treated as multibacillary leprosy.
Despite the conflict with respect to the duration of multidrug therapy,
a 6-month regimen with dapsone and rifampicin is recommended in
case of pausibacillary leprosy, whereas for the multibacillary forms
a 12-month regimen with dapsone, rifampicin and clofazimine is
requiered, as had been started in our patient [8].
The highest prevalence of leprosy is observed in Southeast Asia
(especially in India and Indonesia), in Latin America and Africa [9].
Itstransmission is only partially understood. Sustained exposure
appears to be a prerequisite for clinically manifest disease, whose
development is genetically controlled, as suggested by genome-wide
association studies [10]. Since the introduction of the multidrug
therapy in the mid-1980s the prevalence of leprosy is substantially
and constantly decreasing. In 2015, a total number of 210.758 new
leprosy-patients were globally registered, of which only 18 were
registered in Europe (8 of them in Spain). In Europe most cases are
imported from countries of high prevalence [3,11]. Due to its long
incubation time (between 3 and 4 years for pausibacillary and longer–
up to 15 years for multibacillary form), leprosy may develop in people
emigrating asymptomatic many years after their emigration [11].
With respect to the positivity of IGRA and in particular of
the Quantiferon-Gold tuberculosis test, several reports have
demonstrated a cross-reactivity of constituent peptides of this
test (ESAT6 and CFP10) between Mycobacterium leprae and
Mycobacterium tuberculosis [12].
Conclusion
Early diagnosis and treatment of leprosy can prevent incapacitating disease, which may also appear early in disease course mainly through the irreversible peripheral neuronal injury. Biggest obstacle towards diagnosing of leprosy in Europe and western countries is its rarity and the consequent low level of clinical suspicion. Especially nowadays, due to the increasing immigration from countries with high disease prevalence, a high index of suspicion is required for early diagnosis of leprosy. To this end the long incubation time of leprosy and thus, the possibility of appearance of the first disease manifestations several months, even years after settlement in a low prevalence country have to be considered.
Compliance with Ethical Standards
This is a report on a single patient. This case report complies with the Declaration of Helsinki. Approval is also obtained from the Ethics committee of the Medical University of Hannover. A written consent form has been obtained from the patient for this case report.
References
- Britton WJ, Lockwood DN. Leprosy. Lancet. 2004;363:1209-19.
- Araujo S, Freitas LO, Goulart LR, Goulart IM. Molecular Evidence for the Aerial Route of Infection of Mycobacterium leprae and the Role of Asymptomatic Carriers in the Persistence of Leprosy. Clin Infect Dis. 2016;63(11):1412-1420.
- Ramos JM, Romero D, Belinchón I. Epidemiology of Leprosy in Spain: The Role of the International Migration. PloS Negl Trop Dis. 2016;10:e0004321.
- Aftab H, Nielsen SD, Bygbjerg IC. Leprosy in Denmark1980-2010: a review of 15 cases. BMC Res Notes. 2016;9:10.
- Massone C, Belachew WA, Schettini A. Histopathology of the lepromatous skin biopsy. Clin Dermatol. 2015;33(1):38-45.
- Pardillo FE, Fajardo TT, Abalos RM, Scollard D, Gelber RH. Methods for the classification of leprosy for treatment purposes.Clin Infect Dis. 2007;44:1096-9.
- Ridley DS, Jopling WH. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966;34:255-73.
- Reibel F, Cambau E, Aubry A. Update on the epidemiology, diagnosis, and treatment of leprosy. Med Mal Infect. 2015;45:383-93.
- Global leprosy update, 2015: time for action, accountability and inclusion. Wkly Epidemiol Rec. 2015;91:405-20.
- Alter A, Grant A, Abel L, Alcaïs A, Schurr E. Leprosy as a genetic disease. Mamm Genome. 2011;22:19-31.
- Massone C, Brunasso AM, Noto S, Campbell TM, Clapasson A, Nunzi E. Imported leprosy in Italy. J Eur Acad Dermatol Venereol. 2012;26:999- 1006.
- Rendini T, Levis W. Quantiferon-Gold Tuberculosis Test Cannot Detect Latent Tuberculosis in Patients With Leprosy. Clin Infect Dis. 2015;61:1439-40.