Case Report
Interstitial Ectopic Pregnancy, Diagnosis and Management: A Case Report and Literature Review
Ahmed Samy Yassin1* and Manal Shaker Taha2
1Department of Gynecology, Sidra Medical and Research Center, Doha, Qatar
2Department of Gynecology, Women’s Hospital, Hamad Medical Corporation, Doha, Qatar
*Corresponding author: Ahmed Samy Yassin, Sidra Medical and Research Center, Al Nasr Tower, PO Box 26999, Doha, Qatar
Published: 06 May, 2017
Cite this article as: Yassin AS, Taha MS. Interstitial Ectopic Pregnancy, Diagnosis and Management: A Case Report and Literature Review. Ann Clin Case Rep. 2017; 2: 1352.
Abstract
In this review we describe the incidence, presentation, available methods for early diagnosis and the different treatment options for management of interstitial ectopic pregnancy and how to distinguish this type of ectopic pregnancy from other pregnancies that implant laterally in the uterus. A case of viable interstitial ectopic pregnancy treated with intramuscular methotrexate is presented and used to critically discuss its management in the light of the presented evidence and we concluded by highlighting some practical points in reporting, diagnosing and managing interstitial ectopic pregnancy.
Introduction
Many authors in the published literature use the terms ‘‘cornual’’ and ‘‘interstitial’’ pregnancy interchangeably for pregnancies implanted laterally in the uterus [1-3]. Others reserved the term cornual pregnancy to describe pregnancy in one horn of a bicornuate uterus or a septate uterus or in a rudimentary horn of a unicornuate uterus [4,5]. These terms are also frequently confused with the term angular pregnancy, in which the pregnancy is also implanted laterally in the uterus but the implantation is medial to utero-tubal junction and the round ligament of the uterus [5]. Some authors consider the term angular pregnancy is obsolete as it simply refers to a normal intrauterine pregnancy that happens to be located laterally within the uterine cavity and be carried to term [6]. While other considers this type of pregnancy to either develop directly over the tubal opening or more probably, in the interstitial portion of the tube immediately external to that opening and then grows into the adjacent uterine cavity, thus considering angular pregnancy as a continuation of an interstitial pregnancy [7,8]. Angular pregnancy can progress to second trimester or even to term but is associated with high rates of spontaneous abortion, uterine rupture, and placenta accreta, with rupture occurring in as many as 23.5% of all angular pregnancies [9].
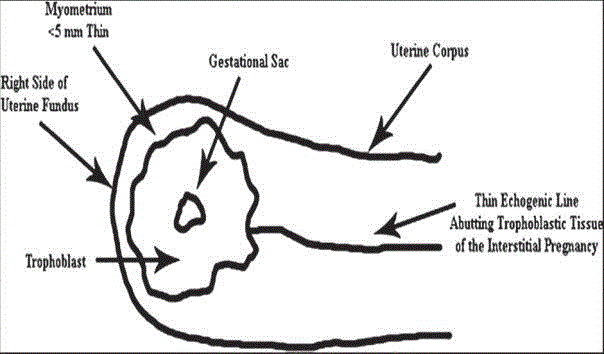
Figure 1
Figure 2
Figure 2
Diagram depicting the sonographic criteria for diagnosis of
interstitial pregnancy in the first trimester. A gestational sac eccentrically
located in the right side of the uterine fundus, and the interstitial line sign,
which refers to an echogenic line that runs from the endometrial cavity to the
cornua of the uterus abutting the gestational sac [3].
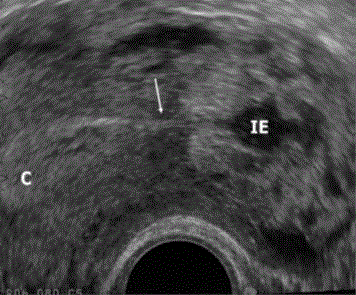
Figure 3
Figure 3
Ultrasound findings of Interstitial ectopic pregnancy (IE), Interstitial
line sign is seen (arrow) joining the endometrial cavity (c) [6].
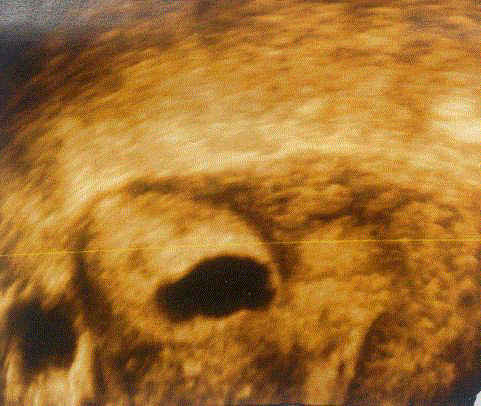
Figure 4
Figure 4
A 3-D ultrasonography of the uterus showing gestational sac
embedded in the right cornua. Uterine cavity and musculature are also seen
clearly [20].
Definitions and Anatomy
Anatomically, the interstitial part of the fallopian tube (the intramural portion) is 0.7 mm wide
and approximately 1 cm - 2 cm long with a slightly tortuous course within the muscular wall of the
uterus extending obliquely upward and outward from the uterine cavity. Therefore, an interstitial
ectopic pregnancy is defined as pregnancy implanted in the interstitial portion of the fallopian tube
that is within the muscular wall of the uterus, lateral to the round ligament of the uterus (Figure 1) [1,2,10].
Incidence
The incidence of interstitial pregnancy varies between 2 to 4% of all ectopic implantations and
one in every 2500-5000 live births [2,11-14]. The interchangeable use of the terms cornual, angular and interstitial pregnancy in literatures may have created problems in the reported incidence of
interstitial ectopic pregnancy. The frequency of interstitial ectopic pregnancy is increasing steadily
with the increasing use of assisted reproductive technologies [10,15]. The predisposing factors for interstitial ectopic pregnancies include: pelvic inflammatory disease, previous ipsilateral or bilateral
salpingectomy, previous ectopic and pelvic surgery, tumours, uterine anomalies and in vitro
fertilization [16].
Delayed diagnosis of interstitial pregnancy is the main factor contributing to the high maternal
mortality rate in comparison to that for tubal ectopic pregnancies; the mortality rate for tubal
ectopic pregnancy was reported to be 0.14%, whilst that for interstitial pregnancy was reported to be
nearly 15 times higher, at 2%-2.5% [11].
Presentation
Interstitial ectopic pregnancy can present with the classic
symptoms of ectopic pregnancy namely, amenorrhoea, vaginal
bleeding, and abdominal pain, but the last two symptoms may not
arise in some un-ruptured interstitial ectopic pregnancies which
could remain asymptomatic for several weeks because the interstitial
portion of the tube can expand more than other tubal segments
before it ruptures [17].
In a retrospective review of 109 cases of interstitial ectopic
pregnancy 54.5% of patients presented with abdominal pain, 22.7%
with vaginal spotting and 20.5% were asymptomatic [18].
Because interstitial ectopic pregnancy is located in the upper
lateral area of the uterus where there is extensive uterine-ovarian
vessels anastomosis it results in catastrophic haemorrhage when
ruptured [16]. This explains the higher mortality rate of this type of
ectopic pregnancy and highlights the need for early diagnosis.
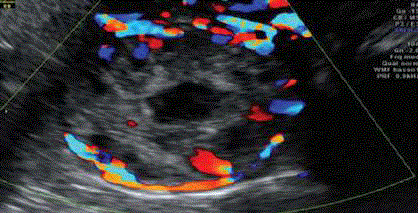
Figure 5
Figure 5
Doppler US showing interstitial gestational scan surrounded by
intense peripheral vascularization around the interstitial gestation [17].
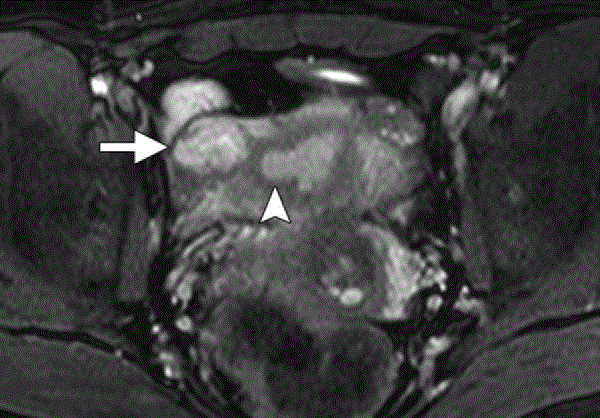
Figure 6
Figure 6
MR image of right Interstitial ectopic pregnancy showing a
heterogeneous intensity mass (arrow) arising from the interstitial portion
of the right fallopian tube immediately lateral to the right uterine angle
(arrowhead). Notice the intact junctional zone between the uterine cavity and
the mass [5].
Diagnosis
Interstitial ectopic pregnancy remains one of the most difficult
gestations to diagnose. Eccentrically located intrauterine gestational
sac can be misinterpreted as interstitial pregnancy on ultrasound
evaluation. Even when interstitial pregnancy is suspected on
ultrasound scan (US), laparoscopic findings may be negative in early
stages and present a diagnostic dilemma [19,20].
US identification of the interstitial portions of the two tubes is
very helpful to exclude the diagnosis of unicornuate uterus and
is essential for making the diagnosis of interstitial pregnancy. By
examining the fundal aspect of the uterus in a transverse section, the
interstitial tubes can be identified as thin hyper echoic lines extending
from the lateral aspect of the uterine cavity through the myometrium
towards the uterine serosa [21]. They can be seen routinely in women
with intrauterine pregnancies <7 weeks’ gestation. In women with
extra-uterine pregnancies, the uterine position and size are usually
not affected by the growth of pregnancy and therefore it is possible
to identify the interstitial segments of the tube as late as the second
trimester of pregnancy [6].
US findings consistent with interstitial gestation include an
eccentrically located gestational sac at the superior fundal level of
the uterus at least 1 cm from the lateral edge of the uterine cavity
surrounded by asymmetrical thin (<5 mm) myometrial tissue with
a distinct and separate empty uterine cavity, and the ‘‘interstitial
line sign’’, an echogenic line that extends into the upper regions
of the uterine horn and borders the margin of the intramural gestational sac, representing either interstitial portion of the tube or endometrium, which depends on the age and size of the gestation
[3,22]. The interstitial line sign (Figure 2) have 80% sensitivity and
98% specificity for the diagnosis of interstitial ectopic pregnancy.
However these criteria are reproducible only in the first trimester and
the diagnosis become more difficult and equivocal when the gestation
enlarges in the second trimester [2,3,23].
An editorial suggested adaptation of two ultrasound findings for
diagnosing interstitial ectopic pregnancy [6]: 1) visualization of the interstitial line adjoining the gestational sac and the lateral aspect
of the uterine cavity; and 2) the continuation of myometrial mantle
around the ectopic sac (Figure 3).
A recent review of 11 cases of interstitial pregnancies found
that the presence of a cornual mass in the absence of any evidence
of an intrauterine pregnancy is the most significant finding with a
sensitivity of 80%. However, they acknowledged that sometimes it
can be difficult to distinguish between interstitial pregnancy and a
tubal ectopic pregnancy on two dimensional ultrasound scan [19].
Three-dimensional ultrasonography (3D scan) was shown to be
an important diagnostic tool as it may depict precise location of the
interstitial gestational sac differentiating it from eccentrically located
intrauterine gestational sac as compared to other imaging modalities
(Figure 4) [20]. The use of colour flow Doppler in combination with
scan may reveal the presence of a vascular ring, which proved an
intense peripheral vascularization around the interstitial gestation
(Figure 5) [17].
Magnetic Resonance Imaging (MRI) can also be used in nonurgent
cases and for evaluation of cases in which ultrasound scans
have been inconclusive. The main advantage of MRI as compared
with ultrasound is the ability to visualize the whole uterus, and thus
identify the exact site of implantation. The gestational sac is located
eccentrically in the uterine fundus and is surrounded by asymmetric
thin myometrium. Distinction between an angular pregnancy and
an interstitial pregnancy can sometimes be difficult. However, in
early interstitial pregnancy the presence of an intact junctional zone
between the uterine cavity and the lesion (the gestation) is suggestive
of an interstitial location (Figure 6) [5,23]. In second trimester the visualization of the decidua adjacent to the eccentrically located
gestational sac is highly suggestive of interstitial pregnancy and help
to eliminate the diagnosis of angular pregnancy [23]. MRI features of
advanced interstitial ectopic pregnancy include eccentric location of
the gestational sac, covered by asymmetric thin myometrium on the
uterine fundus, and clear visualization of the decidua adjacent to it
[23]. MRI may also show myometrial defect and hemoperitoneum
which are signs of uterine rupture.
Differential diagnosis
Diagnosis can be delayed due to the difficulty of distinguishing
an interstitial pregnancy from other laterally implanted pregnancies
including an eccentrically located intrauterine gestational sac (Table 1). In early pregnancy, 3D scan can be utilized to help identifying the
precise location of the gestational sac [20].
Differential diagnosis with an angular pregnancy is acritical
aspect. While an interstitial pregnancy is implanted in the uterine
portion of the tube, angular pregnancy is an intrauterine pregnancy
located near the utero-tubal junction. Anatomically, these two ectopic
pregnancies cause a different uterine distortion: Usually the angular
pregnancy leads to a bulge medial to the round ligament, and the
interstitial one determines a lateral bulge [17]. Clinically, given the
intrauterine location of angular pregnancies as described above and
the enveloping myometrium, these patients are likely to present with
symptoms later than patients with other types of ectopic pregnancies.
On US examination the myometrium is thicker in angular pregnancies
and has at least 5 mm or more of myometrium surrounds all sides
of the sac while in interstitial pregnancies it is thinner <5 mm and
present only in the supero-lateral portion of the sac [9,17].
At MRI, angular pregnancy appears as gestational sac implanted
in the lateral angle of the uterus and can be confused with normal
pregnancies. This gestational sac will be completely surrounded by
uterine myometrium, although focal thinning is sometimes seen
(Figure 7). High signal intensity in the uterine wall itself can represent
intramural haemorrhage. It is also important to look for placental
invasion of the myometrium because placenta accreta is frequently
seen in these patients. Given the propensity for rupture, it is also
important to look for myometrial discontinuity and hemoperitoneum
whenever angular pregnancy is suspected [5].
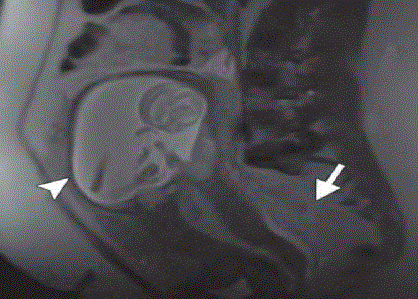
Figure 7
Figure 7
MRI showing second trimester gestational sac with thinning of
the overlying myometrium (arrowhead) and intermediate-signal-intensity
hemorrhage (arrow) in the cul-de-sac [5].
Figure 8
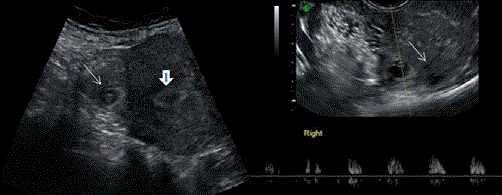
Figure 8
On the left Abdominal scan showing gestational sac with fetal pole
in the right side of the uterine fundus ( thin white arrow) distant from the
endometial cavity ( arrow head). On the right TV scan showing the same
gestational sac with demonstration of fetal heart beats and interstitial line
sign (thin white arrow).
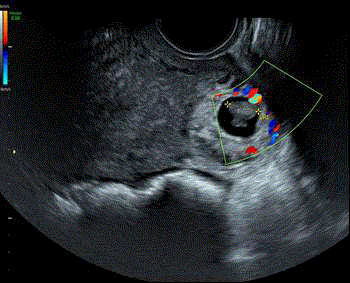
Figure 9
Figure 9
TV scan with colour flow Doppler demonstrating reduced peripheral
vascular flow surrounding the right cornual ectopic pregnancy.
Treatment
Medical management with methotrexate has been used as first-line treatment in appropriate cases. A strict inclusion criteria
(early gestation, diameter <4 cm, serum beta Human Chorionic
Gonadotropin (hCG) of <10,000 IU/l, no evidence of rupture) was
suggested and treatment was shown to be success in cases that satisfy
these criteria and in women who are able to be monitored for a quite
long time after treatment and treated further if required [17].
When the interstitial pregnancy is correctly identified at an early
stage, and is un-ruptured, medical management can be attempted,
with the potential advantage of avoiding surgery and with an increased
likelihood of preserving fertility. Systemic injection of methotrexate
(MTX) is the most extensively studied medical regimen. This drug
has been administered either a single dose or in a multiple-dose fashion with reasonable success and also administered in a localized
fashion, with both US and laparoscopic guidance [24]. The systemic
route of administration offers advantages over local injection of the
ectopic gestation in that it is less invasive and not operator dependent.
Follow-up may need to be prolonged after medical treatment of
interstitial pregnancy as the initial hCG values tend to be higher than
those encountered with tubal ectopic pregnancy [17]. Other medical
treatments showing some success include potassium chloride and
prostaglandins, both injected directly into the gestational sac during
ultrasonographic visualization.
Medical treatment of ectopic pregnancy has a failure rate of up
to 35% and rupture of the ectopic pregnancy is also possible during methotrexate treatment even in the face of decreasing hCG values [25-27]. Surgery is indicated when patient refuse, failed or unsuitable
for medical treatment, in the presence of significant symptoms, in
ruptured cases or in large interstitial ectopic with the presence of a
heartbeat [19].
In the past, laparotomy with either hysterectomy or cornual
resection was advocated. Recently, options such as laparoscopic
cornuostomy (incision of the cornual region), or cornuectomy
(resection of the cornual region of the uterus and the suturing of the
incision site) have been reported [18,19]. The current trend is to use
conservative surgical alternatives to cornual resection in an attempt to
increase future fertility and decrease the risk of uterine rupture during
a subsequent pregnancy [1]. These conservative surgical treatments
successfully used combination of hysteroscopic, laparoscopic and
ultrasound guided transcervical evacuation of interstitial ectopic
pregnancy [28,29].
Intraoperative bleeding remains a major problem for laparoscopic
management of interstitial ectopic pregnancies, because of the rich
supply of blood vessels at the location of the interstitial ectopic
pregnancy [18].
Various haemostatic techniques have been attempted for
the control of bleeding. These have included intracorporeal and
extracorporeal suturing, endoloop or encircling suture before
evacuation of conception, electrosurgey in addition to diluted
vasopressin injection, fibrin glue, a harmonic scalpel and uterine
artery embolization [18,30-34]. Successful laparoscopic management
of interstitial ectopic pregnancies using the automatic stapler to
simultaneously excise and stitch the uterine cornua has also been
reported [35].
One of the key challenges after laparoscopic surgery is the
persistent trophoblastic activity even after cornual resection which
may be due to increased myometrial invasion in some interstitial
ectopic pregnancies. This has been treated successfully with
methotrexate [11,19].
Prognosis
The mortality rate for interstitial pregnancy was reported to
be 15 times more than the mortality rate of tubal ectopic; this was
attributed to delayed diagnosis of interstitial pregnancy but may also
be due to confusion with angular and cornual pregnancies which tend
to rupture at a later stage [11,17,23].
Persistently high serum Beta hCG after laparoscopic management
has been treated with methotrexate [31]. Recurrence of interstitial
ectopic pregnancy after medical or laparoscopic treatment including
conservative laparoscopic surgery or laparoscopic corneal resection
has been reported [18,31].
Ruptured uterus during subsequent pregnancy has been
reported after spontaneous resolution and after surgical treatment
of interstitial pregnancy [36,37]. The drawback to cornual resection
and suturing is the use of a full-thickness uterine wall incision (as in
hysterotomy) and when electrosurgery is used; the depth of a thermal
damage is difficult to be assessed. This has prompt many surgeons
to recommend elective caesarean delivery in subsequent pregnancies
to decrease the risk of uterine rupture [19,37]. However, successful
vaginal delivery have also been reported after both cornual resection
with suturing of the uterine defect and laparoscopic cornuostomy in
which the uterine wall was left open for secondary healing [31,38,39].
Table 1
Table 1
The diagnostic criteria of different types of laterally implanted pregnancies which can be confused with interstitial pregnancy
Case Presentation
A 37 years old women, G8P6+1 presented after 6 weeks of amenorrhea to the ultrasound department. US showed 10 mm gestational sac containing viable fetal pole with a crown rump length (CRL) of 3.4 mm corresponding to 6 weeks gestation. The gestational sac was seen at the right cornual aspect of the uterus and a diagnosis of right sided viable cornual ectopic was reported (Figure 8). She was admitted for further assessment. She was asymptomatic with normal stable vital signs, her hemoglobin was 13.2 mg/dl and her hCG level was 8735 mIU/ml. In view of viable cornual pregnancy and high hCG levels the patient was counselled re surgical treatment as the first option but she declined that option. Medical treatment with intramuscular methotrexate was discussed and she opted for that. She agreed to stay in hospital during medical treatment and have surgery only if developed pain, her vital signs deteriorate and her hemoglobin dropped. She was kept at hospital. According to unit protocol she was given 90 mg Intramuscular (IM) methotrexate and remained stable with no pain in the hospital until day 4 after methotrexate injection. Her serum hCG increased to 13469 mIU/ml. She remained asymptomatic with normal vital signs and no change to her haemoglobin levels till day 7. Her hCG on day 7 dropped to 10137 mIU/ml. A repeat US showed re-demonstration of right sided cornual pregnancy with CRL equivalent to 7 weeks gestation but no fetal heart beat was detected with the presence of Sub chorionic hematoma measuring 1.0 cm x 0.3 cm and reduced peripheral vascularity on colour flow Doppler (Figure 9). In view of the good response to IM methotrexate with a drop of >15% in hCG level and the US findings it was agreed to discharged her for outpatient monitoring and follow up. She was warned and advised to report immediately to the hospital if she developed any pain. She had 6 outpatient visits for serial hCG levels which continue to drop till it become < 5 mIU/ml on day 47 after methotrexate injection. She was discharged from follow up.
Discussion
The case presented was successfully managed with systemic methotrexate. This case report and other have confirmed the successful use of methotrexate in treating early interstitial ectopic pregnancies [1,2,24,25]. The presence of fetal heart beats in this case confirmed that medical treatment can be used successfully in early viable interstitial ectopic pregnancy even in the presence of detectable fetal heart beats with serum beta hCG of over 8000 mIU/ml. This is in contrast to other papers suggesting that the presence of fetal heart beat is a contraindication to medical management [19]. In the case presented US did not report any of the sonographic diagnostic criteria described in the literatures above for diagnosing interstitial ectopic pregnancy [2,3,6,23]. There was no use of the available 3D US to confirm diagnosis and the term Cornual instead of interstitial ectopic was used. However, the use of colour flow Doppler during follow up and demonstration of reduced flow and vascularity around the gestational sac was helpful to encourage continuation with medical management. The case reported declined surgical treatment and agreed to stay inpatient after the medical treatment until it was felt safe to discharge her for outpatient monitoring. She had 6 weekly visits over 47 days after the IM methotrexate until she was discharged from follow up. This prolonged follow up requires patient motivation and may not be suitable to all women.
Conclusion
The term cornual pregnancy should not be used to descript
interstitial ectopic pregnancy in literatures. Researchers and
editors should adhere to this so the true incidence and the different
management modality outcomes for interstitial ectopic pregnancy
can be accurately reported.
Sonographers should be encouraged to look for the described the
sonographic diagnostic criteria and document these in their report
to increase clinician’s confidence with the diagnosis especially in
asymptomatic patients with viable pregnancy and high serum beta
hCG levels. If 3D scan is available this should also be used as it may
increases the diagnostic accuracy.
Women suitable for medical management of interstitial ectopic
pregnancy should be informed that medical management with
systemic methotrexate for early viable interstitial ectopic pregnancy
can be successful but post treatment follow up can be prolonged and
they need to agree to that before offering the treatment.
The available literature do not provides agreed guidance regarding
future pregnancy risks and optimum mode of delivery following
treatment of interstitial ectopic pregnancy.
References
- Tinelli A, Malvasi A, Pellegrino M, Pontrelli G, Martulli B, Tsin DA. Laparoscopical management of cornual pregnancies: a report of three cases. Eur J Obstet Gynecol Reprod Biol. 2010;151(2):199-202.
- Ross R, Lindheim SR, Olive DL, Pritts EA. Cornual gestation: a systematic literature review and two case reports of a novel treatment regimen. J Min Inv Gynecol. 2006;13:74-8.
- Ackerman TE, Levi CS, Dashefsky SM, Holt SC, Lindsay DJ. Interstitial line: sonographic finding in interstitial (cornual) ectopic pregnancy. Radiology. 1993;189(1):83-7.
- Mavrelos D, Sawyer E, Helmy S, Holland TK, Ben-Nagi J, Jurkovic D. Ultrasound diagnosis of ectopic pregnancy in the non-communicating horn of a unicornuate uterus (cornual pregnancy). Ultrasound Obstet Gynecol. 2007;30(5):765-70.
- Parker RA, Yano M, Tai AW, Friedman M, Narra VR, Menias CO. MR imaging findings of ectopic pregnancy: a pictorial review. Radiographics. 2012;32(5):1445-60.
- Jurkovic D, Mavrelos D. Catch me if you scan: ultrasound diagnosis of ectopic pregnancy. Ultrasound Obstet Gynecol. 2007;30(1):1-7.
- Baldawa PS, Chaudhari HK. Angular ectopic pregnancy presenting as rupture of lateral wall of the uterus. J Hum Reprod Sci. 2008;1(1):33-4.
- MOIR JC. “Angular pregnacy”. In: KEER J M, MOIR J C, editors. Operative obstetrics. 7th ed. London: Balliere Tindall & Cox; 1964. 733-98.
- Biswas SP, Halder S, Shirin FB. Angular Pregnancy Presenting as Cornual Ectopic Pregnancy. Medicine Today. 2014;26(01):63-5.
- Bouyer J, Coste J, Fernandez H, Pouly JL, Job-Spira N. Sites of ectopic pregnancy: a 10-year population-based study of 1800 cases. Hum Reprod. 2002;17:3224-30.
- Tulandi T, Al-Jaroudi D. Interstitial pregnancy: results generated from the Society of Reproductive Surgeons registry. Obstet Gynecol. 2004;103:47-50.
- Stovall TG, McCord ML. Early pregnancy loss and ectopic pregnancy. In: Berek JS, Adashi EY, Hillard PA, editors. Novak’s Gynecology. 12th ed. Baltimore: Williams and Wilkins; 1996. 487-524.
- Tulandi T, Saleh A. Surgical management of ectopic pregnancy. Clin Obstet Gynecol. 1999;42(1):31-8.
- Faraj R, Steel M. Review Management of cornual (interstitial) pregnancy. TOG. 2007;9(4):249-55.
- Rock JA, Jones HW, Te Linde RW. Te Linde’s. Operative gynecology. 10th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2008.
- Lau S, Tulandi T. Conservative medical and surgical management of interstitial ectopic pregnancy. Fertil Steril. 1999;72:207-15.
- Corioni S, Perelli F, Bianchi C, Cozzolino M, Maggio L, Masini G. Interstitial pregnancy treated with a single-dose of systemic methotrexate: A successful management. J Res Med Sci. 2015;20:312-6.
- Hwang JH, Lee JK, Lee NW, Lee KW. Open cornual resection versus laparoscopic cornual resection in patients with interstitial ectopic pregnancies. EJOG. 2011;156:78-82.
- MacRae R, Olowu, Rizzuto MI, Odejinmi F. Diagnosis and laparoscopic management of 11 consecutive cases of cornual ectopic pregnancy. Arch Gynecol Obstet. 2009;280:59-64.
- Singh N, Tripathi R, Mala Y, Batra A. Diagnostic Dilemma in Cornual Pregnancy- 3D Ultrasonography may Aid!! J Clin Diagn Res. 2015;9(1):QD12-3.
- Timor-Tritsch IE, Farine D, Rosen MG. A close look at early embryonic development with the high-frequency transvaginal transducer. Am J Obstet Gynecol. 1988;159:676-81.
- Timor-Tritsch IE, Monteogudo A, Matera C, Veit CR. Sonographic evolution of cornual pregnancies treated without surgery. Obstet Gynecol. 1992;79:1044-9.
- Hamouda ESM, Littooij AS, Thia EW, Ong CL. Ruptured Interstitial Ectopic Pregnancy at 18 Weeks Gestation Diagnosed by MRI: A Case Report. JRCR. 2013;7(10):34-42.
- Barnhart K, Spandorfer S, Coutifaris C. Medical treatment of interstitial pregnancy. A report of three unsuccessful cases. J Reprod Med. 1997;42:521-4.
- Tang A, Baartz D, Khoo SK. A medical management of interstitial ectopic pregnancy: a 5-year clinical study. Aust N Z J Obstet Gynaecol. 2006;46(2):107-11.
- Grobman WA, Milad MP. Conservative laparoscopic management of a large cornual ectopic pregnancy. Hum Reprod. 1998;13:2002-4.
- Voigt RR, van der Veen F, Karsdorp VH, Hogerzeil HV, Ketting BW. Treatment of interstitial pregnancy with methotrexate: report of an unsuccessful case. Hum Reprod. 1994;9(8):1576-9.
- Thakur Y, Coker A, Morris J, Oliver R. Laparoscopic and ultrasound-guided transcervical evacuation of cornual ectopic pregnancy: an alternative approach. Journal of Obstetrics and Gynaecology. 2004;24(7):809-10.
- Pal B, Akinfenwa O, Harrington K. Hysteroscopic management of cornual ectopic pregnancy. BJOG. 2003;110(9):879-80.
- Deruelle P, Lucot JP, Lions C, Robert Y. Management of interstitial pregnancy using selective uterine artery embolization. Obstet Gynecol. 2005;106:1165-7.
- Ng S, Hamontri S, Chua I, Chern B, Siow A. Laparoscopic management of 53 cases of cornual ectopic pregnancy. Fertil Steril. 2009;92(2):448-52.
- Moon HS, Choi YJ, Park YH, Kim SG. New simple endoscopic operations for interstitial pregnancies. Am J Obstet Gynecol. 2000;182(1 Pt 1):114-21.
- Morita Y, Tsutsumi O, Momoeda M, Taketani Y. Cornual pregnancy successfully treated laparoscopically with fibrin glue hemostasis. Obstet Gynecol. 1997;90:685-7.
- Dalkalitsis N, Stefanidis K, Paschopoulos M, Navrozoglou I, Mouzakioti E, Lolis D. Laparoscopic treatment of interstitial pregnancy using the harmonic scalpel. Clin Exp Obstet Gynecol. 1998;25:49-50.
- Sergent F, Le Cornec JB, Meilhaud MF, Marpeau L. Laparoscopic cornual excision with an automatic stapler for ruptured interstitial pregnancies. J Gynecol Obstet Biol Reprod. 2003;32:426-30.
- Downey GP, Tuck S. Spontaneous uterine rupture during subsequent pregnancy following non-excision of an interstitial ectopic gestation. BJOG. 1994;101:162-3.
- Auamkul S. Rupture of gravid uterus after cornual resection. A case report. J Reprod Med. 1970;5(5):218-20.
- Tulandi T, Vilos G, Gomel V. Laparoscopic treatment of interstitial pregnancy. Obstet Gynecol. 1995;85(3):465-7.
- Pansky M, Bukovsky I, Golan A, Raziel A, Caspi E. Conservative management of interstitial pregnancy using operative laparoscopy. Surg Endosc. 1995;9:515-6.