Case Report
Pelvic Congestion Syndrome: Diagnostic Challenge and Endovascular Treatment
Marc Salomon*, Jessica Goldman and Sohail Contractor
Department of Radiology, Rutgers New Jersey Medical School, Newark, USA
*Corresponding author: Marc Salomon, Department of Radiology, Rutgers New Jersey Medical School, 185 S Orange Ave, Newark, NJ 07103, USA
Published: 25 Apr, 2017
Cite this article as: Salomon M, Goldman J, Contractor S. Pelvic Congestion Syndrome: Diagnostic Challenge and Endovascular Treatment. Ann Clin Case Rep. 2017; 2: 1344.
Abstract
Pelvic congestion syndrome (PCS) is comprised of a constellation of symptoms including noncyclical pelvic pain, pelvic varicosities, dysmenorrhea, and dyspareunia. There is a higher incidence of PCS in young, multiparous, pre-menopausal women in the age range of 20-40 years. Symptoms worsen through the day and are exacerbated by standing and increased physical activity. Patients often experience relief in supine position. The diagnosis of PCS should be considered when a premenopausal multiparous woman presents with pelvic pain of greater than 6 months’ duration and is found to have pelvic varices on non-invasive imaging (MRV, transvaginal ultrasound). The diagnosis is typically confirmed by venography demonstrating dilatation of and reflux within the ovarian vein, which occurs more commonly on the left side due to its drainage into the left renal vein (often considered the female equivalent of scrotal varicoceles). Ovarian vein venography and embolization to prevent further reflux is the first-line treatment with resolution of symptoms seen in 70-90% of patients. Here, we report the case of a patient who presented with the classic signs of PCS and underwent ovarian vein embolization therapy.
Case Presentation
A 35-year-old G4P4 woman presented to the interventional radiology clinic for evaluation
of chronic pelvic pain. Her pain initially developed three years ago following the delivery of her
last child and became progressively more severe. Her pain was localized to the mid-suprapubic
and perineal regions. The pain was exacerbated by standing up and alleviated by lying supine. She
also complained of deep pelvic pain during intercourse. In addition, she reported having palpable
blood vessels in the pelvic and perineal regions. Her daily activities were limited as a result of her
symptoms.
The patient previously had varicose vein sclerosis in her left lower extremity, which partially
improved her symptoms. However, she intermittently continued to experience symptoms consistent
with venous distension. After addressing her concern about her symptoms with her gynecologist,
pelvic ultrasound was performed and demonstrated no evidence of fibroids or other adnexal
pathology. Physical exam at the time of presentation was negative for venous prominence in the
abdomen and pelvis. However, there were some dilated veins noted over the left inner thigh and
vulvar area. There was no tenderness over the abdomen and pelvis.
Because the patient’s presentation was worrisome for pelvic congestion syndrome, she was
advised to undergo angiographic venogram of the pelvis and possible ovarian vein embolization
in case of significant reflux. The left renal and ovarian veins were accessed and catheterized via the
right common femoral vein. Venograms demonstrated significant reflux of contrast from the left
renal vein into the left ovarian vein. The entire left renal vein and the left ovarian vein were dilated
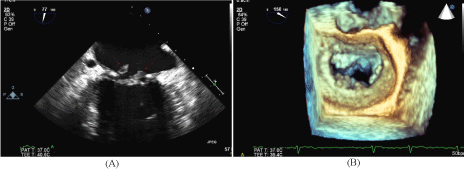
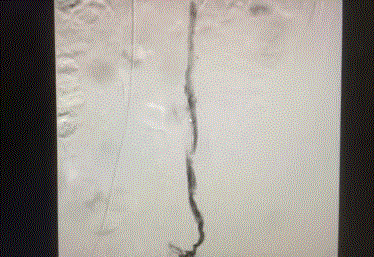
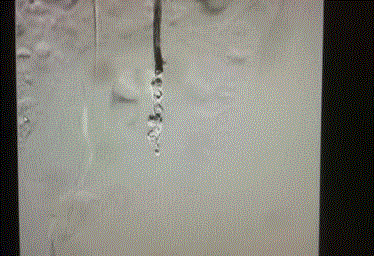
and opacified up to the level of the ovaries. Figures 1-4 show significant contrast reflux from the left
renal vein into the left ovarian vein down to the level of the ovaries.
Numerous collaterals were observed along the dilated venous channel. On the basis of the
patient’s symptoms and significant radiographic findings, embolization of the left ovarian vein
using several VortX-185-12 mm microcoils (Boston Scientific, Natick, MA),was performed.
Approximately 7 cm length of vein was embolized. Embolization extended from the iliac brim to the
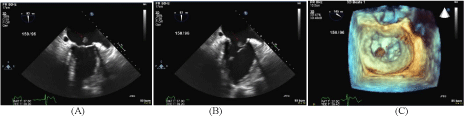
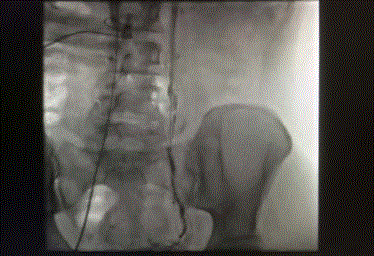
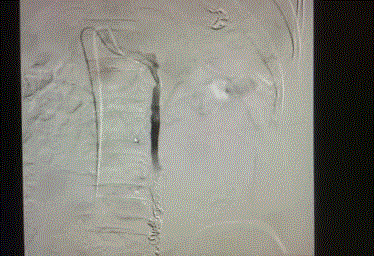
level of L2 vertebral body. Figures 5-7 reveal embolization of the left ovarian vein using microcoils
reaching the level of L2 vertebral body.
A subsequent venogram exhibited no further reflux to the level of the ovaries. Right ovarian
venogram and bilateral internal iliac venograms were then performed which demonstrated no significant pelvic collaterals or reflux back to the uterus and ovaries.
She was discharged home that same evening and was seen in a
subsequent clinic follow up visit at 4 weeks. Her pain is down from
a score of 8/10 to 2/10. She states her lifestyle is much improved and
she is able to perform her daily activities without pain.
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
Figure 6
Discussion
Chronic pelvic pain (CPP) represents a common complaint
amongst young multiparous women. Establishing a cause for
CPP remains a diagnostic challenge, as the differential diagnosis
is vast. Disorders of the gynecologic, urinary, gastrointestinal,
musculoskeletal, and neurological systems may give rise to CPP.
Endometriosis, uterine leiomyomata, pelvic inflammatory disease,
interstitial cystitis, irritable bowel syndrome, and pelvic neuralgia are
among the common causes of CPP, but the differential may include
many more conditions. In fact, the cause of CPP remains undiscovered
in up to 61% of women, even after significant radiological and
laparoscopic investigation [1-4,5]. Pelvic congestion syndrome (PCS) should be considered in a young multiparous woman that presents
with non-cyclical CPP for over 3 months. Patients will often complain
of pelvic varicosities, abdominal/pelvic tenderness, and CPP that
worsens with long periods of standing and is relieved by lying supine.
A multi-disciplinary approach to the workup, involving Gynecology,
Urology, Vascular Surgery, and Interventional Radiology, is often
necessary to exclude other conditions and confirm the diagnosis
in patients with PCS [1,4]. Anatomically, there are two distinct
venous channels that give rise to pelvic venous insufficiency (PVI),
the ovarian veins and the internal iliac veins. The ovarian veins are
often comprised of multiple channels themselves, rather than solitary
vessels. Additionally, they collateralize with the retroperitoneal
and ascending lumbar veins. These two features require extensive
embolization of a significant length of the ovarian veins as the
retrograde reflux can reconstitute into the various channels/
collaterals. The internal iliac veins receive blood flow from the uteroovarian,
hemorrhoidal, sacral, and vesicular venous beds and are also
responsible for pelvic varices.PCS most commonly involves the left
ovarian vein, due to its drainage into the left renal vein, and the right internal iliac vein [6].
Ovarian vein incompetence is thought to occur in up to 10%
of women and about 60% of those women will develop PCS. The
pathophysiological mechanism by which it develops is considered
multifactorial with genetics and pregnancy to be significant
contributors. During pregnancy, increased blood volume and
blood flow in the pelvis combined with mechanical compression
from an enlarged uterus results in valvular injury and permanent
dilatation of the pelvic veins, resulting in retrograde reflux. The same
pathophysiological mechanism is implicated in varicose veins of the
lower extremities, as well [6]. In fact, due to venous connections
between the pelvis and lower extremities, it is possible that lower
extremity varicose veins refractory to endovascular treatment can
be treated the same way as PCS, with embolization of the internal
iliac and ovarian veins. Additionally, it is important to distinguish
increased blood flow into the ovaries from retrograde reflux into the
ovaries. PCS must be a condition caused by the latter in order for
ovarian vein embolization to be a successful treatment.
Diagnostic workup often includes abdominal/transvaginal
ultrasound, CT/MRI, and venography. On CT/MRI, the findings
of ovarian vein diameter >8 mm and 4 or more parauterine veins
with a vein diameter of >4 mm support a PCS diagnosis. However, a recent study published by Dos Santos et al. indicates that there is no significant difference in diameter between competent and refluxing
veins and therefore techniques that measure venous diameter may not
be suitable for diagnosis of PVI [7]. CT/MRI are effective in excluding
secondary causes of PVI, including Nutcracker syndrome and May-
Thurner syndrome. However, it should be noted that because PVI
is partially alleviated by lying supine, CT/MRI may lack sensitivity
in detecting mild-moderate cases of PVI [6]. Laparoscopy is not
considered a first-line diagnostic tool for PCS as CO2 insufflation can
cause venous distension and PCS has reportedly been detected in only
20% of cases [1,4,8].
A variety of treatment options exist for PCS, including hormonal
therapy (medroxyprogesterone and gonadotropin-releasing hormone
analogs), operative management (surgical ligation of ovarian veins),
and endovascular treatment. However, endovascular treatment has
become the gold standard of therapy due to its effectiveness and
minimal side-effect profile. The American Venous Forum guidelines
recommend coil embolization and transcatheter sclerotherapy for
confirmed PCS, with other treatment methods reserved for refractory
cases [6].
A 2014 comprehensive review of 13 studies published between 1966
and July 2014 was conducted by Hansrani “et al.” [9] With 866 cases
of transvenous-occlusion therapy for CPP reviewed. Interventions
included femoral or jugular vein access to the ovarian veins and
insertion of metallic coils, sclerosants, or glue; most of the cases used
either metallic coils alone or foam sclerotherapy in combination with
metallic coils. Subjective improvements in pain ratings were seen in
all of the studies including in pelvic pain frequency, dysmenorrhea,
and dyspareunia. There was a reported 4-17% symptom recurrence
during the 1-5 year post-procedure period in five of the studies.
There were 4 successful pregnancies reported post-embolization.
Complete occlusion of veins that showed reflux was accomplished in
98-100% of cases. Mean right ovarian vein diameter, as measured by
transvaginal ultrasound, reduced from 4.5mm to 3.19mm and mean
left ovarian vein diameter reduced from 6.3mm to 4.5mm six months
after sclerotherapy. Notably, there was no significant difference found between success rates and occlusion material used. The procedure
was found to be safe with few significant complications reported.
Reported complications included 0.6% perforations or injuries to the
target vein and 1.6% coil migration to the pulmonary artery or renal
circulation. No long term complications or death were found in any
of the studies.
Figure 7
Figure 8
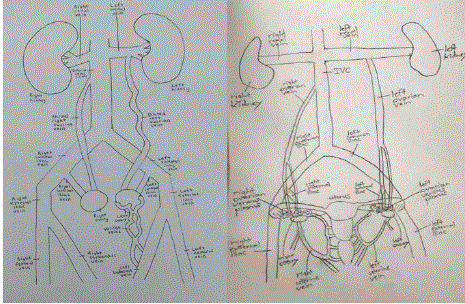
Figure 8
Schematic of pelvic venous anatomy showing tortuous ovarian venous channels that give rise to varices in PCS.
Conclusion
Despite several studies reported on PCS, further research is necessary in order to establish diagnostic criteria for the condition. At this time, a consensus has not yet been established on the specific symptoms and the degree of venous distention required to make the diagnosis. Additionally, the pathophysiological relationship between CPP and pelvic venous insufficiency remains poorly understood. Prospective controlled trials remain necessary to establish a strong causal relationship between CPP and PVI [9]. Although it appears that ovarian vein embolization is effective in treating patients with CPP and PVI, the etiology responsible for the pain and the mechanism by which the treatment works are areas that require investigation [3].
References
- Mahmoud O, Vikatmaa P, Aho P, Halmesmäki K, Albäck A, Rahkola-Soisalo P et al. Efficacy of endovascular treatment for pelvic congestion syndrome. Journal of Vascular Surgery: Venous and Lymphatic Disorders. 2016;4(3):355–70.
- Durham JD, Machan L. Pelvic congestion syndrome. Semin Intervent Radiol. 2013;30(4):372-80.
- Champaneria R, Shah L, Moss J, Gupta JK, Birch J, Middleton LJ, et al. The relationship between pelvic vein incompetence and chronic pelvic pain in women: Systematic reviews of diagnosis and treatment effectiveness. Health Technol Assess. 2016;20(5):1-108.
- Borghi C, Dell'Atti L. Pelvic congestion syndrome: the current state of the literature. Arch Gynecol Obstet. 2016;293(2):291-301.
- Daniels JP, Khan KS. Chronic pelvic pain in women. BMJ. 2010;341:c4834.
- Koo Sonya, Chieh-Min Fan. Pelvic Congestion Syndrome and Pelvic Varicosities. Tech Vasc Interv Radiol. 2014;17(2):90–5.
- Santos SJ Dos, Jm Holdstock, CC Harrison, AJ Lopez, MS Whiteley. Ovarian Vein Diameter Cannot Be Used as an Indicator of Ovarian Venous Reflux. Eur J Vasc Endovasc Surg. 2015;49(1):90–4.
- Tu FF, Hahn D, Steege JF. Pelvic congestion syndrome-associated pelvic pain: a systematic review of diagnosis and management. Obstet Gynecol Surv. 2010;65(5):332-40.
- Hansrani Vivak, Abeera Abbas, Sahil Bhandari, Ann-Louise Caress, Mourad Seif, Charles N Mccollum. Trans-venous occlusion of incompetent pelvic veins for chronic pelvic pain in women: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2015;185:156–63.