Case Report
Mirror Syndrome: A Novel Cause of Shortness of Breath in the Third Trimester of Pregnancy
Emilie J Calvello Hynes1* and Rouda Nuaimi2
1Department of Emergency Medicine, University of Colorado, USA
2Department of Internal Medicine and General Surgery, Dubai Medical College, UAE
*Corresponding author: Emilie J Calvello Hynes, Department of Emergency Medicine, University of Colorado, Room 711, Leprino Office Building, Campus Box B215, 12401 E 17th Ave, 7th Floor, Aurora, CO, 80045 USA
Published: 03 Apr, 2017
Cite this article as: Hynes EJC, Nuaimi R. Mirror
Syndrome: A Novel Cause of Shortness
of Breath in the Third Trimester of
Pregnancy. Ann Clin Case Rep. 2017;
2: 1320.
Abstract
Mirror syndrome is a rare association of fetal and placental hydrops with maternal pre-eclampsia and edema. We report a case of a 32 years old pregnant female at 28 weeks of gestation with hydrops fetalis who presented to our Emergency Department with severe shortness of breath, elevated blood pressure and bilateral lower limb edema. The patient underwent bedside ultrasound, which showed pulmonary edema and bilateral pleural effusions with normal cardiac function. The patient was diagnosed with Mirror Syndrome and underwent an emergency cesarean section delivery. The case illustrates a novel cause of shortness of breath in the third trimester that is an indication for emergent obstetric consult and delivery.
Keywords: Mirror Syndrome; Preeclampsia; Hydrops fetalis; Edema
Introduction
Dyspnea during pregnancy is a common complaint; approximately 60% -70% of women will have dyspnea with no previous history of cardiopulmonary disease [1,2]. The mechanical effect of 20 the enlarging gravid uterus and the superior displacement of diaphragm, is counterbalanced by hormonally induced increased excursion of the rib cage [1-4]. Progesterone mediated hyperventilation is thought to be due to the increased sensitivity of respiratory center to PaCO2, which increases the minute ventilation and the tidal volume [2,3,5]. The pregnant woman subjectively perceives this hyperventilation as shortness of breath [5]. The cardiovascular changes in pregnancy of increased blood volume that progresses throughout gestation as well as increased cardiac output may also contribute to the subjective sense of dyspnea [6]. Dyspnea in pregnancy can also be secondary to pathological illness including inflammatory lung disease, sepsis, pulmonary emboli, pulmonary edema, preeclampsia, peripartum cardiomyopathy and pneumothorax requires early recognition and intervention [7]. Asthma exacerbation that requires medical intervention occurs in about 20% of pregnant patients with approximately 6% necessitating inpatient admission [8]. Pneumonia occurs in less than 1.5% of pregnant women, but is often severe enough to cause mortality, secondary to the change in maternal immunity that occurs in pregnancy to protect the fetus [7,9]. Preeclampsia affects 2-8% of all pregnancies of which 3-10% have pulmonary edema and secondary respiratory distress [7,10]. Peripartum cardiomyopathy, a rare serious congestive heart failure with unclear etiology, has wide incidence that varies among geographic region from an incidence of 1:4000 in the United States to 1:100 in Nigeria [11-13]. Amniotic fluid embolism is rare but has high mortality rate due to its difficulty in effective treatment; fifty-one percent of cases will have respiratory symptoms and 24% will have pulmonary edema. The classic patient will present with sudden onset of dyspnea, with hypoxia and cardiovascular collapse [14]. Finally, the incidence of pulmonary embolism is five times more common than non-pregnant women, secondary to the hypercoagulable state that occurs during pregnancy [7]. It is essential for emergency physician to differentiate physiological dyspnea from dyspnea associated with pathological diseases. History of abrupt paroxysmal dyspnea is more likely to be due to pathological etiology especially when patient has other signs and symptoms such as edema, fever, headache, elevated blood pressure, or abdominal pain. Increase in respiratory rate greater than 20 cycles/min, arterial blood PaCO2 less than 30 mm Hg or greater than 35 mm Hg, hypoxemia, abnormal forced expiratory spirometry values, or echocardiography may also raise the probability of pathological dyspnea [9]. We present a case of mirror syndrome which is a rare syndrome that presents with undifferentiated dyspnea and respiratory distress but mandates emergent obstetric consult and delivery.
Case Presentation
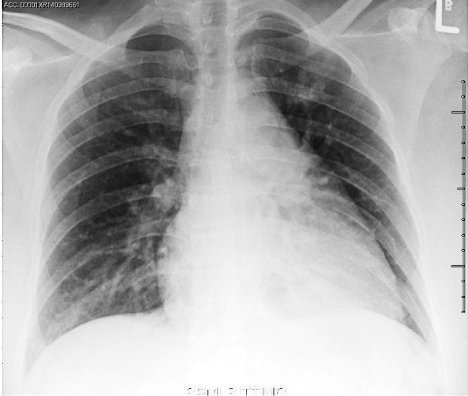
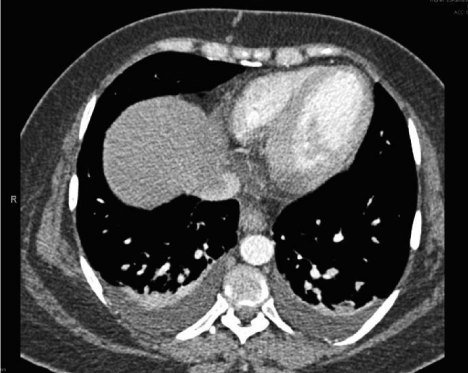
A 32 years old pregnant female with history of iron deficiency anemia presented to our Emergency Department (ED) at 28 weeks of gestation with a chief complaint of shortness of breath and bilateral limb edema. She had been diagnosed with hydrops fetalis at an ultrasound 4 weeks prior to presentation. The patient reported her symptoms developed gradually over the past 4 weeks, and had worsened over the last 2 days accompanied by orthopnea and paroxysmal nocturnal dyspnea. The patient also reported an elevated blood pressure for the last 3 weeks but no intervention had been made by her obstetrician. She denied history of fever, cough, hemoptysis, nausea, vomiting, headache, visual change, dysuria or history of DVT. There were no contractions, leakage of fluid, vaginal bleeding or abdominal pain. She had one previous successful pregnancy that was complicated by gestational diabetes but never hypertension or preeclampsia. Three of her siblings had congenital heart disease and another two had cerebral palsy. Upon arrival in the ED, her vital signs revealed a blood pressure of 148/90 mm Hg, heart rate of 90 beats/minute, respiratory rate 30 cycles/minute and an oxygen saturation of 99% on room air. On initial examination, patient appeared anxious, dyspneic in moderate respiratory distress. She had pink conjunctiva and normal sclera color. Her neck was supple with midline trachea and no distended neck veins. Auscultation of the chest revealed clear lungs with good air entry and fine bilateral basilar rales with decreased breath sounds at the bases. Cardiovascular exam showed a regular rate and rhythm with no murmurs, rubs, or gallops. The patient had soft nontender abdomen with a gravid uterus, and a fundal height corresponding to 30 weeks of gestation. Lower extremities were symmetrically enlarged with +3 pitting edema and intact pulses. During her initial ED evaluation, a bedside ultrasound was preformed to assess patient’s cardiovascular status. Echocardiography (ECHO) revealed a normal ejection fraction with symmetric wall contractility, minimal pericardial effusion and no RV dilatation, hypertrophy or tamponade physiology. A pulmonary ultrasound showed no evidence of pneumothorax but did reveal bilateral multiple pulmonary B-lines and pleural effusions. The abdominal evaluation showed a gravid uterus with a viable fetus, polyhydramnios and minimal free fluid in Morrison’s pouch and the splenorenal recess. Laboratory investigations indicated a hemoglobin level of 8.8 g/dl, with low hematocrit and mean corpuscular volume. The platelet count, LDH, uric acid, cardiac markers and coagulation profile were all within normal limits. Liver enzymes were slightly elevated (AST 44 IU/L, Alkaline Phosphatase 176 IU/L) and magnesium level was low (0.69 mmol/L). The electrocardiogram revealed normal sinus rhythm, normal intervals without signs of ischemic changes. Chest X-ray exhibited bilateral pulmonary vascular congestion (Figure 1). Computed tomography (CT) scan of the chest showed no pulmonary embolus but bilateral pleural effusions with pulmonary edema (Figure 2). The patient was diagnosed as having Mirror Syndrome with preeclampsia based on her clinical signs and symptom in the presence of known fetal hydrops. In the ED, she received magnesium sulfate, labetalol for blood pressure control and furosemide for gentle diuresis. The patient was admitted to the obstetric team for urgent management and consideration of early delivery. Formal echocardiography was performed that agreed with the primary findings of the ED ECHO. Cardiology consultation suggested no cardiac etiology for her presentation. The obstetric team proceeded with cesarean delivery after giving appropriately timed steroids and sufficient counseling to the patient and her husband. Post-operatively, the patient was observed for 24 hours in the intensive care unit and then discharged on day 3 to routine follow up with no overt complications and resolution of her initial symptoms of dyspnea. Her child died within 24 hours secondary to severe respiratory distress and autopsy of the fetus was not offered due to religious reasons.
Figure 1
Figure 2
Discussion
Our patient presented with dyspnea and generalized edema in third trimester of pregnancy, with elevated blood pressure and proteinuria. The differential diagnosis in this case scenario included preeclampsia, abruption of placenta with secondary amniotic fluid embolism, pulmonary thrombotic emboli, peripartum cardiomyopathy with secondary pulmonary edema and HELLP syndrome. Pneumonia and pneumothorax was excluded by history, clinical exam, pulmonary ultrasound and chest X-ray. CT scan and ECHO excluded peripartum cardiomyopathy, and pulmonary embolism. Because of hypertension, anemia and proteinuria, HELLP syndrome was considered, but a normal LDH level, normal platelet count and liver enzymes less than two-time the upper limit made HELLP less likely [15]. Mirror syndrome was first described by John W. Ballantyne in 1892 when he reported a case of maternal edema in pregnancy with fetal and placental hydrops secondary to rhesus isoimmunization [16,17]. Only few cases were subsequently published and were limited to pregnancy with hydrops fetalis secondary to rhesus isoimmunization. In late 1970s, with the development of ultrasound, the enhanced ability to assign prenatal diagnoses helped in describing cases with Mirror syndrome that were associated with non-immune related hydrops pregnancies. In addition to rhesus isoimmunization, Mirror syndrome has been reported in twintwin transfusion syndrome, cases of viral infections (specifically parvovirus B19, cytomegalovirus and Coxsackie B virus), associated fetal malformations and with fetal or placental tumors [16]. Mirror syndrome is a rare diagnosis with unclear pathogenesis that can occur anytime during the antepartum period and may continue postpartum [17,19-21]. Common features of Mirror syndrome include maternal hypertension, generalized edema, and proteinuria accompanied with fetal hydrops and placental enlargement [21]. In 80-100% of reported cases, edema was the maternal key sign while hypertension (57-78%) and proteinuria (20-56%) were also associated indicators. Severe maternal complications including pulmonary edema were only present in 21.4% of cases [16]. Significant overlap in signs and symptoms exist between Mirror syndrome and preeclampsia but distinguishing features are the presence of fetal hydrops, low hematocrit and polyhydramnios [17]. In absence of well established guideline regarding diagnosis and management of Mirror syndrome and based on published cases, maternal symptoms will resolve either by treating the cause of fetal hydrops, death of the fetus, or delivery. An example of spontaneous reversal of maternal symptoms occurred in a twin gestation where the affected twin died and reversal of symptoms occurred within 24 hour with a good outcome for both mother and the other fetus [22-23].
Conclusion
Mirror syndrome is a frequently under diagnosed rare disorder that has high association with increased fetal mortality and maternal morbidity. Early recognition of this syndrome is essential, as reversal of maternal symptoms requires early aggressive intervention. Medical management might be transitorily helpful but the definitive management is delivery of fetus and placenta. Emergency physicians should consider Mirror syndrome early in their differential diagnosis of dyspnea in the pregnant patient, particularly with signs of edema, hemodilution and fetal hydrops to provide the best outcomes for the patient and fetus.
References
- Prowse CM, Gaensler EA. Respiratory and acid-base changes during pregnancy. Anesthesiology. 1965;26:381-92.
- Gee JB, Packer BS, Millen JE, Robin ED. Pulmonary mechanics during pregnancy. J Clin Invest. 1967;46(6):945-52.
- Milne JA. The respiratory response to pregnancy. Postgrad Med J. 1979;55(643):318-24.
- Turner AF. The chest radiograph in pregnancy. Clin Obstet Gynecol. 1975;18(3):65-74.
- McCormack MC, Wise R.A. Respiratory physiology in pregnancy. in: Ghada Bourjeily, Karen Rosene-Montella (Eds.) Pulmonary problems in pregnancy. New York: Humana Press; 2009:19-26.
- Carson M, Rosene-Montella K. Common Cardiac Complaints in Pregnancy. Women’s Health in Primary Care. 1999;2(7):533– 44.
- Malhotra N, Puri R, Malhotra J. (Eds). Pulmonary Disease in Pregnancy. in: Donald School, Manual of Practical Problems in Obstetrics 1st edition. London: Jaypee Brothers Medical Ltd. 2012;265–78.
- Murphy VE, VL Clifton, PG Gibson. Asthma exacerbations during pregnancy: incidence and association with adverse pregnancy outcomes. Thorax. 2006;61(2):169–76.
- Lim WS, Macfarlane JT, Colthorpe CL. Pneumonia and pregnancy. Thorax. 2001;56(5):398-405.
- Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376(9741):631-44.
- Lampert MB, Lang RM. Peripartum cardiomyopathy. Am Heart J. 1995;130(4):860-70.
- Neligan PJ, Laffey JG. Clinical review: Special populations--critical illness and pregnancy. Crit Care. 2011;15(4):227.
- Sliwa K, Damasceno A, Mayosi BM. Epidemiology and etiology of cardiomyopathy in Africa. Circulation. 2005;112(23):3577-83.
- Frati P, Foldes-Papp Z, Zaami S, Busardo FP1. Amniotic fluid embolism: what level of scientific evidence can be drawn? A systematic review. Curr Pharm Biotechnol. 2014;14(14):1157-62.
- Padden MO. HELLP syndrome: recognition and perinatal management. Am Fam Physician. 1999;60(3):829-36, 839.
- Dunn PM. Dr John Ballantyne (1861-1923): perinatologist extraordinary of Edinburgh. Arch Dis Child. 1993;68(1):66-7.
- van Selm M, Kanhai HH, Gravenhorst JB. Maternal hydrops syndrome: a review. Obstet Gynecol Surv. 1991;46(12):785-8.
- Braun T, Brauer M, Fuchs I, Czernik C, Dudenhausen JW, Henrich W, et al. Mirror syndrome: a systematic review of fetal associated conditions, maternal presentation and perinatal outcome. Fetal Diagn Ther. 2010; 27(4):191–203.
- Reiss HE. Historical insights: John William Ballantyne 1861-1923. Hum Reprod Update. 1999;5(4):386-9.
- Steven A Ordorica, Frances Marks, Faith J Frieden, Iffath A Hoskins, Bruce K Young, et al. Aneurysm of the vein of Galen: a new cause for Ballantyne syndrome. Am J Obstet Gynecol. 1990;162(5):1166–1167.
- Gedikbasi A, Oztarhan K, Gunenc Z, Yildirim G, Arslan O, Yildirim D, et al. Preeclampsia due to fetal non-immune hydrops: mirror syndrome and review of literature. Hypertens Pregnancy. 2011;30(3):322-30.
- Kontomanolis EN, Lambropoulou M, Tsagias N, Koutlaki N, Limperis A, Galazios G, et al. The riddle of Ballantyne's syndrome in the aspect of hydrops fetalis. J Matern Fetal Neonatal Med. 2014;27(11):1172-3.
- Pirhonen JP, Hartgill TW. Spontaneous reversal of mirror syndrome in a twin pregnancy after a single fetal death. Eur J Obstet Gynecol Reprod Biol. 2004;116(1):106-7.