Case Report
Fine Needle Aspiration Cytology and DNA Analysis by Flow- Cytometry of Atypical Medullary Breast Carcinoma: A Case Report
Trutin Ostović K1*, Čikara I2, Žic R3, Lipšanski Z4 and Lambaša S5
1Department of Clinical Cytology, Dubrava University Hospital, Zagreb, Croatia
2Department of Radiology, Dubrava University Hospital, Zagreb, Croatia
3Department of Plastic, Reconstructive and Esthetic Surgery Dubrava University Hospital, Zagreb, Croatia
4Department of Molecular Biology, Clinical Unit of Cytology, Dubrava University Hospital, Zagreb, Croatia
5Department of Pathology, Dubrava University Hospital, Zagreb, Croatia
*Corresponding author: Trutin Ostović K, Department of Pathology and Cytology, “Dubrava” University Hospital, Avenija Gojka Šuška 6, 10000 Zagreb, Croatia
Published: 23 Mar, 2017
Cite this article as: Trutin Ostović K, Čikara I, Žic R,
Lipšanski Z, Lambaša S. Fine Needle
Aspiration Cytology and DNA Analysis
by Flow- Cytometry of Atypical
Medullary Breast Carcinoma: A Case
Report. Ann Clin Case Rep. 2017; 2:
1313.
Abstract
Atipycal medullary carcinoma is a rare breast carcinoma (less than 4%patients). We report a case of fine needle cytology of atypical medullary carcinoma. It was diagnosed in 43-years-old woman 18 years ago. She also suffered from Morbus Hashimoto and hypothireosis. The tumour was detected by mammography in the upper part of the left breast. Fine needle aspiration cytology with ultrasound guidance of the tumour was performed. Cytological diagnosis of poorly differentiated carcinoma of the breast was put. The patient was operated on (mastectomy) and histological diagnosis of atypical medullary carcinoma was put. The tumour was oestrogen and progesterone receptor negative. It was hyper dyploid with high proliferative activity by flow cytometric DNA analysis. The patient was without any sign of the tumour 15 years after the operation when she died because of the heart failure.
Introduction
Medullary carcinoma of the breast is a rare tumour (4%-7% patients). Atypical medullary carcinoma is more rare (less than 4% patients). It is considered as distinctive subtype with a more favourable prognosis than invasive ductal carcinomas and other histologic subtypes despite it’s an aplastic morphology [1-4]. There is no specific pathological reason for this name of the tumour. It is named according its appearance which resembles the tissue of the medulla oblongata region of the brain [5]. This term medulla derives from the Latin root “medulla” that means “marrow” [5]. Atypical medullary carcinoma of the breast has similar prognostic factors as medullary carcinoma but survival is better [4,6]. It is usually diagnosed in younger female patients (age of 35 or younger) without family history1.It differs from typical medullary carcinoma in terms of pathologic figures with an infiltrative margin, mild mononuclear infiltration, a low nuclear grade and presence of an intraductal component [3,6]. Cystic type is diagnosed in premenopausal women or menopausal women [6]. It can be detected in men as well [5]. It is not hereditary as ductal breast carcinoma, but according to some authors the atypical medullary carcinoma has the same clinical and pathological characteristics and outcomes with those of the invasive ductal carcinoma [1]. The tumour tends to be smaller than 3 cm with circumscribed margins and firm consistency. Therefore it can be mistaken clinically and radiologically for benign lesion. Axillary lymph nodes are often enlarged and they are not metastatic but reactive [1]. The prognosis is poor because 90% of the tumours are oestrogen and progesterone receptor negative [1]. Histological criteria for typical medullary carcinoma by Ridolfi are: predominantly syncytial growth pattern in more than 75% tumour area, circumscription with a pushing margin, moderate to marked lymphoplasmacytic infiltration, poorly differentiated nuclear grade with a high mitotic rate, scant stroma and absence of an intraductal component [3]. Although atypical medullary carcinoma has some morphological characteristics similar as typical medullary carcinoma, it is different because it has: infiltrative margin, mild mononuclear infiltration, a low nuclear grade and presence of an intraductal component, mild or negligible mononuclear infiltrate or infiltrate at margins only; nuclear grade 3 (higher than for medullary carcinoma) and presence of microglandular features (opposite to medullary carcinoma) [3,4]. Oestrogen and progesterone receptor negative tumours are 90 % [1]. Genetically medullary breast carcinoma is often associated with BRCA-1 oncogene mutations and TP53 alterations [7,8]. They are usually aneuploid or tetraploid with disputable S-phase fraction value because of high intra-tumour heterogeneity [9].
Figure 1
Figure 2
Figure 2
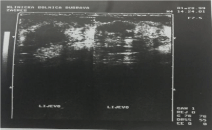
Ultrasonography: indistinct lesion with inhomogeneous,
hypoechoic texture in cystic tissue of the left breast.
Case Presentation
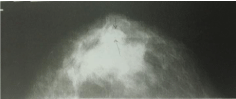
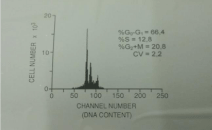
A 43-year-old woman, a mother of 22-years old daughter, presented with fibrocystic disease 18 years ago. Besides she suffered of Morbus Hashimoto and hypothireosis and therefore was cured with Vobenol (100 mg per day for 4 years). Mammography showed uniformly dense, non-calcified, round, well-circumscribed mass in the upper outer quadrant of the left breast (Figure 1). Ultrasonography showed indistinct lesion with inhomogeneous, hypoechoic texture in cystic tissue (Figure 2). Fine needle aspiration cytology with ultrasound guidance was performed using 23 gauge needle and 20 ml disposable syringe. Cytological diagnosis of poorly differentiated carcinoma was put. The patient has undergone surgery (mastectomy) and histological diagnosis of atypical medullary carcinoma was put. Flow cytometric DNA analysis was performed from few parts of the tumour tissue. It showed aneuploidy–hyperdiploidy (DI = 1, 3; three peaks in G0-G1 phase) and high proliferative activity (PI = 33, 6%; S-phase fraction = 12, 8 %) (Figure 3). We did not perform detection of BRCA 1 gene in that time. The patient was treated with postoperative chemotherapy (CMF protocol) and radiotherapy. She remained free of tumour 15 years after surgery when she died because of the heart failure.
Figure 3
Figure 3
DNA analysis by flow cytometry: aneuploid-hyperdiploid (three
peaks) with high proliferative activity S-phase fraction 12.8%).
Figure 4
Figure 4
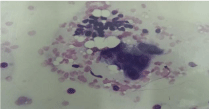
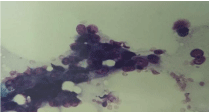
Cytological smear: cluster of benign small epithelial cells with scant
cytoplasm (upper part) and cluster of large poorly diferentiated malignant
epithelial cells (May-Grunwald Giemsa /MGG/ 400x).
Figure 5
Figure 6
Figure 6
Cytological smear: malignant cells are rather large with
anysocariosis, prominent nucleoli and ill-defined cytoplasm (MGG 400x).
Results and Discussion
Cytology
We had to be very cautious in cytological analysis of the smears
because Vobenol can cause morphological changes to the benign
epithelial cells and they can be very similar to malignant cells. The
smears were hyper cellular. Groups of benign rather small epithelial
cells with scant cytoplasm were intermixed with large, moderately
to poorly differentiated malignant epithelial cells distributed
individually or in loose syncytial groupings and clusters (Figure 4).
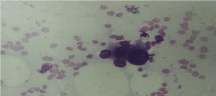
Malignant cells were rather large (more than 25 μm in diameter),
slightly anisokaryotic and pleomorphic (Figure 5). The nuclei had
irregular nuclear membrane and chromatin was dense and fine, often
granuloreticular. Nucleoli were prominent, enlarged and usually
multiple with irregular shapes and the cytoplasm was greyish-blue,
often ill-defined, and scant to abundant (Figure 4 and 6). Apparent
vacuolation was not true: aspect was caused by scattered fat droplets
(Figure 4 and 5). Large naked tumour nuclei, some bizarre, with rare
mitosis, few lymphocytes and stromal cells could occasionally be seen
(Figure 4 and 5). Therefore we put the cytological diagnosis of poorly
differentiated carcinoma.
The fact that it is impossible to put the accurate cytological
diagnosis of atypical medullary carcinoma is perhaps the reason why
I could not find any cytological paper about it but the author have
found only rare papers about cytology of medullary carcinoma [8,10
-12].
Figure 7
Figure 7
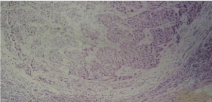
Large sheets of malignant epithelial cells with syncytial growth
pattern and dense fibrous stroma infiltrated by lymphocytes (HE100x).
Figure 8
Pathology
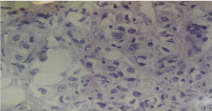
Well-circumscribed soft tumour mass with bulging surface measured 2 cm × 1.5 cm. Seventeen axillary lymph nodes were free of tumour. Tumour had predominantly syncytial growth pattern (over 75 % of the tumour). There were large sheets of malignant epithelial cells with syncytial growth pattern and dense fibrous stroma infiltrated by lymphocytes (Figure 7). Malignant cells have vesicular pleomorphic nuclei and prominent nucleoli (Figure 8). Tumour was oestrogen and progesterone receptor negative and katepsin D slightly positive.
DNA Analysis
DNA analysis was made by flow cytometry using program PARA l by EPICS-C flow-cytometer. Cytological material is adequate for DNA flow cytometric analyses (DNA content can be performed on 10000–2000 cells and regular fine needle sampling contains approximately 100000 cells [11]. Despite that, we have prepared suspension of single nuceli prepared from paraffin wax embedded blocks of the tumour because the results are better than from the fresh tissue because of intra-tumour heterogeneity [9]. Therefore we prepared cells from six slices for each paraffin block. The suspension of single nuclei was prepared from paraffin wax embedded blocks of the tumour because the results are better than from the fresh tissue because of intra-tumour heterogeneity [9]. We prepared six slices for each paraffin block–a 30 micron section was cut, washed in xylene to remove the paraffin. After that it was rehydrated by repeat treatment with decreasing concentrations of ethanol. Mechanical disaggregation with metal mesh was performed and followed by enzymatic dispersion with pepsin. Then the samples were filtered through nylon mesh and stained with propidium iodide containing ribonuclease. Cellular DNA content was measured using program PARA I by EPICS-C flow-cytometer. Tumour was aneuploidhyperdiploid with high proliferative activity (DNA index was 1,3; there were three peaks in G0-G1 phase, P index was 33,6%; S phase fraction was 12,8%) (Figure 3).
Conclusion
Ultrasound guided FNAC of impalpable lesions of the breast is very useful in searching and diagnosing of breast carcinoma. It is difficult to put the exact cytological diagnosis of atypical medullary carcinoma of the breast because the lymphocytes are not present in the smears or if there are only few lymphocytesin the smears. Therefore pathohistological analysis was required for the definitive accurate diagnosis of atypical medullary carcinoma of breast in our patient.
References
- Park I, Kim J, Kim M, Bae SY, Lee SK, Kil WH, et al. Comparison of the characteristics of medullary breast carcinoma and invasive ductal carcinoma. J Breast Cancer. 2013;16(4):417-25.
- Bloom HJ, Richardson WW, Field JR. Host resistance and survival in carcinoma of breast: a study of 104 cases of medullary carcinoma in a series of 1,411 cases of breast cancer followed for 20 years. Br Med J. 1970;3(5716):181-8.
- Ridolfi RL, Rosen PP, Port A, Kinne D, Miké V. Medullary carcinoma of the breast: a clinicopathologic study with 10 year follow-up. Cancer. 1977;40(4):1365-85.
- Foschini MP, Eusebi V. Rare (new) entities of the breast and medullary carcinoma. Pathology. 2009;41(1):48-56.
- Steven Halls (Moose and Doc): Breast Cancer visited on: January 25th. 2017.
- Mateo AM, Pezzi TA, Sundermeyer M, kelley CA, Klimberg VC, Pezzi CM. Atypical medullary carcinoma of the breast has similar prognostic factors and survival to typical medullary breast carcinoma: 3976 cases from the National Cancer Data Base. J Surg Oncol. 2016;114(5):533-6.
- Ashida A, Fukutomi T, Tsuda H, Akashi-Tanaka S, Ushijima T. Atypical medullary carcinoma of the breast with cartilaginous metaplasia in a patient with a BRCA1 germline mutation. Jpn J Clin Oncol. 2000;30(1):30- 2.
- Galzerano A, Rocco N, Accurso A, Ciancia G, Campanile AC, Caccavello F, et al. Medullary breast carcinoma in an 18-year-old female: report on one case diagnosed on fine-needle cytology sample. Diagn Cytopathol. 2014;42(5):445-8.
- Bergers E, van Diest PJ, Baak JP. Tumour heterogeneity of DNA cell cycle variables in breast cancer measured by flow cytometry. J Clin Pathol. 1996;49(11):931-7.
- Aouni NE, Athanasiou A, Mansouri D, Marsiglia H, Mathieu MC, Suciu V, et al. Medullary breast carcinoma: a case report with cytological features and histological confirmation. Diagn Cytopathol. 2006;34(10):701-3.
- El-Nagar AK. Viehl Ph: Solid tumour DNA content analysis- Chapter 20: Flow cytometry protocols, in book Methods in molecular biology“, Volume 263 of the series; 2 ed. by Hawley TS, Hawley RF (eds. Humana Press, 2nd Edition by Hawley TS and Hawley RF): 2001; 355-70.
- Akbulut M, Zekioglu O, Kapkac M, Ozdemir N. Fine needle aspiration cytologic features of medullary carcinoma of the breast: a study of 20 cases with histologic correlation. Acta Cytol. 2009;53(2):165-73.