Case Report
Pseudomonas Sepsis Associated with Skin and Cardiac Involvements in an Infant with Congenital Nephrotic Syndrome
Mitra Naseri1*, Mohammad- Reza Naghibi2 and Elham Samezghandi1
1Department of Pediatric Nephrology, Mashhad University of Medical Sciences, Iran
2Department of Pediatric Cardiology, Mashhad University of Medical Sciences, Iran
*Corresponding author: Mitra Naseri, Associate Professor, Department of Pediatric Nephrology, Dr. Sheikh, Children Hospital, Mashhad University of Medical Sciences, Mashhad, Iran
Published: 03 Mar, 2017
Cite this article as: Naseri M, Naghibi M-R, Samezghandi
E. Pseudomonas Sepsis Associated
with Skin and Cardiac Involvements
in an Infant with Congenital Nephrotic
Syndrome. Ann Clin Case Rep. 2017;
2: 1286.
Abstract
Introduction: Ecthyma gangrenosum and infective endocarditis are two rare complications of Pseudomonas Aeruginosa infections.
Case Presentation: Our presenting case is an infant with congenital nephrotic syndrome who was
admitted in the hospital with skin lesions of Ecthyma gangrenosum and manifestations of sepsis.
Blood culture revealed Pseudomonas Aeruginosa infection. In spite of no pathologic sound in heart
examination and dramatically response to antibiotics, echocardiography showed vegetation in right
sided heart associated with mild tricuspid regurgitation.
Conclusion: Cases of bacterial sepsis occurs in patients with implanted venous catheter should be
evaluated for bacterial endocarditis even if there is a dramatically response and no finding suggestive
of cardiac involvement in heart examination.
Keywords: Ecthyma gangrenosum; Infective endocarditis; Pseudomonas sepsis
Introduction
Ecthyma gangrenosum (EG), is a rare invasive cutaneous infection that in majority of cases
caused by Pseudomonas. Aeruginosa (PA) and occurs in 30% of Pseudomonas septicemia [1]. This
lesion is related to life-threatening septicemic infections and has high mortality. Infections of PA
typically seen in immunocompromised patients with severe neutropenia [2], agammaglobulinemic
cases or hypogammaglobinemia [3-4]. The EG lesions characterized by red maculae that progress
to a hemorrhagic bluish bullae which rupture to form a central area of necrosis surrounded by an
erythematous halo. Very rarely this invasive skin lesions may be reported in healthy cases [5-7].
Another rare complication of PA is infective endocarditis (IE) [8]. PA account for <1.8% of bacterial
endocarditis [9]. Most cases of Pseudomonas IE seen in intravenous drugs abusers. Isolated rightsided
pseudomonas IE generally can be managed with antibiotic therapy, with or without valve
surgery [10].
Genetic defects account for the majority of CNS, but especially in developing countries,
congenital infections (syphilis, toxoplasmosis, rubella, hepatitis B, human immunodeficiency and
cytomegalic viruses) can result to CNS. Mutations in genes Nephrin 1 (NPHS1; Finnish type of
CNS), NPHS2, Wilms tumor 1(WT1; Denys-Drash syndrome, Frasier, and WAGR syndromes),
laminin-β2 (LamB2; Pierson syndrome), LamB3 (Herlitz junctional epidermolysis bullosa),
phospholipase C epsilon 1 (PLCE1), LMX1B (nail-patella syndrome) account for genetic forms of
CNS [11].
Case Presentation
Here we present a two-month boy with diagnosis of CNS in first week of life, with negative
serologic tests for congenital infections. At first presentation Serum albumin and Creatinine were
1.8 gr /dl and 0.3 mg/dl respectively, and urine protein to Creatinine (mg/mg) ratio was 305/4.4. No
genetic study was done since they were not available and kidney biopsy was planned to do after age
3 months to provide better information.
He admitted for fever (axillary temperature = 38.5°C), generalized edema, tachypnea, grunting
and poor feeding. A bout one month ago a deep central venous access devices (poly site) was implanted for daily Albumin infusion and furosemide. As there is no
definite treatment except renal transplantation for patients with CNS,
our case never reached remission of proteinuria during treatment.
He didn’t receive anti-aggregation treatment since parents were
unreliable and we concern about risk of hemorrhage. We prefer to
recommend them in cases with history of thrombosis.
Control of vital signs showed systolic blood pressure (BP) of
85 mmHg with no measurable diastolic BP, pulse and respiratory
rates were 150/minute and 55/minute respectively. At presentation
he had nasal flaring without cyanosis and normal heart and lung
examinations. Oxygen saturation was 98% on oxygen and urine output
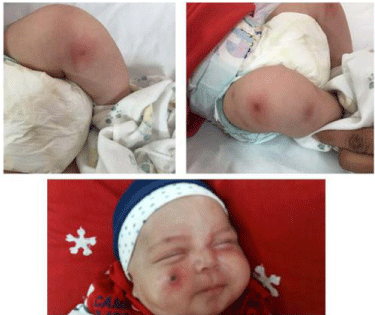
was > 1cc/kg/ hour. Different erythematous macula lesions with a
central necrotic configuration (target sign) were noted in the lower
limbs and face (Figure 1). The lesions were typically EG. After a full
laboratory assessment, treatment with Ceftazidime and Vancomycin
was started. Vancomycin was started to cover staphylococcal
nosocomial sepsis. Laboratory findings are listed in Table 1. Chest
X-ray demonstrated prihilar infiltration with no evidence of bacterial
lung involvement. In second day of admission, respiratory distress
increased (oxygen saturation near to 85% at room), systolic BP
decreased to 70 mmH, so Vancomycin changed to Meropenem and
combination of Meropenem with Ceftazidime selected since PA sepsis was the most probable diagnosis and combination therapy for
PA invasive infections was needed. Also the patient received packed
cell infusion due to anemia. In the day 4 of admission patient had no
respiratory distress and BP returned to normal values. Edema and
induration of skin lesions abruptly decreased and lesions change to
bullae that ruptured and a necrotic lesion at central part remained
(Figure 2).
Blood culture defined growth of Pseudomonas Aeruginosa. At
the day 10, however fever was resolved and there was no abnormal
sound or murmur on heart examination, echocardiography was
done to check bacterial vegetation on heart leaflets. Small vegetation
in septal leaflets of tricuspid valve associated with mild valvar
regurgitation were reported (Figure 3). At the day 12 of admission
patients discharged and recommended to continue the antibiotics
administration through polysite (for 4 weeks) and referee to
cardiologist.
Figure 1
Figure 1
Photograph of Ecthyma gangrenosum shows a dark necrotic
center with surrounding halo. Necrotic lesion on lower extremities and face
(at admission).
Figure 2
Figure 2
Skin lesions in lower extremities hanged to bullous lesions that
ruptured. Erythema surrounding necrotic lesions in lower extremities abruptly
decreased, with no prominent change in facial lesion.
Figure 3
Figure 3
Small vegetation near to septal leaflets of tricuspid valve
(M mode echocardiography), and mild tricuspid regurgitation (Doppler
echocardiography).
Table 1
Table 1
Laboratory findings in our patient at admission and during treatment.
1) Day, 2) Hemoglobin, 3) Hematocrit, 4) Erythrocyte sedimentation rate 1st hour, 5) C-reactive protein, 6) Partial thromboplastin time, 7) prothrombin time, 8) red blood
cells.
Discussion
Congenital nephrotic syndrome (CNS) which characterized
by heavy proteinuria, hypoproteinemia, and edema starts soon
after birth, and is a rare kidney disorder. The loss of the proteins
in the urine leads to hypogammaglobinemia and serum IgG values
commonly is below 2% of normal values in these cases. Urinary losses
of gamma globulin and complement factors B and D prone patients
to infections caused by capsular bacteria such as pneumococci [12].
Prophylactic use of immunoglobulin is not indicated since the infused
immunoglobulin rapidly lost into urine [13].
PA is a common environmental gram-negative bacillus which acts
as an opportunistic pathogen. Almost always P aeruginosa infections
associate with the compromise of host defense mechanisms such as
in acquired immune deficiency syndrome (AIDS), and neutropenic
patients undergoing chemotherapy [11]. Cutaneous manifestations
produced by Pseudomonas infections may be classified into two
groups: primary skin lesions and those in which cutaneous lesions
occur in the context of Pseudomonas bacteremia [14].
In our case it seems that the source of bacteremia was through
central venous catheter. Catheter associated blood stream infections
(CABSI) is a condition that has been reported in 7.6% of cases [15].
Infective endocarditis due to PA is a rare condition and less than
20 cases of P aeruginosa IE involving native valves of non intra
venous drug users have been reported [16]. All of these cases had a
predisposing factor include hemodialysis, cardiac catheterization and
surgery, and gastrointestinal or genitourinary procedures. Our case
had a central venous catheter a condition like that occurs in intra venous drug users and predispose them for blood invasion of bacteria [8].
Our case was an infant with a primary kidney diseases (CNS).
Massive urinary loss of proteins, gamma globulin and complement
factors B and D, and implanting central venous catheter were
predisposing factors for invasive bacterial infections. Study by Harris
in cases with CNS revealed that the serum IgG levels were <25% in
all and most cases had a level <2% of normal infant values [17]. Low
serum gamma globulin levels and deficiency of complement factors
B and D predispose cases to infection with capsular bacteria such as
Streptococcus Pneumonia Other study reported that septic episodes
in infants with CNS mainly caused by staphylococci and coliforms
[13]. Our case is interesting since Pseudomonas infections are rare in
CNS. In addition two rare complications of the infection were found
in our patients, including EG and IE, both are rare in clinical practice
of Pseudomonas aeruginosa infections.
Acknowledgement
The authors would like to appreciate Dr. Sasan for his nice comments in management of the patient.
References
- Bottone EJ, Reitano M, Janda JM, Troy K, Cuttner J. Pseudomonas maltophilia exoenzyme activity as correlate in pathogenesis of ecthyma gangrenosum. J Clin Microbiol. 1986; 24: 995-997.
- Singh TN, Devi KM, Devi KS. Ecthyma gangrenosum: a rare cutaneous manifestation caused by pseudomonas aeruginosa without bacteremia in a leukemic patient--a case report. Indian J Med Microbiol. 2005; 23: 262- 263.
- Almeida JF, Sztajnbok J, Troster EJ, Vaz FA. Pseudomonas aeruginosa septic shock associated with ecthyma gangrenosum in an infant with agammaglobulinemic. Rev Inst Med Trop Sao Paulo. 2002; 44: 167-169.
- Ng W, Tan CL, Yeow V, Yeo M, Teo SH. Ecthyma gangrenosum in a patient with hypogamma- globulinemia. J Infect. 1998; 36: 331-335.
- Athappan G, Unnikrishnan A, Chandraprakasam S. Ecthyma gangrenosum: presentation in a normal neonate. Dermatol Online J. 2008; 14: 17.
- Gençer S , Ozer S, Ege Gül A, Doğan M, Ak O. Ecthyma gangrenosum without bacteremia in a previously healthy man: a case report. J Med Case Reports. 2008; 2: 14.
- Mull CC, Scarfone RJ, Conway D. Ecthyma gangrenosum as a manifestation of Pseudomonas sepsis in a previously healthy child. Ann Emerg Med. 2000; 36: 383-287.
- Venkatesan A, Spalding C, Speedie A, Sinha G, Rumbaugh JA. Pseudomonas aeruginosa infective endocarditis presenting as bacterial meningitis. J Infect. 2005; 51: 199-202.
- Polovina M, Potpara T, Milošević I, Stepanović J, Jovanović M, Pavlović M. Mitral valve endocarditis caused by Pseudomonas Aeruginosa: a case report. J Infect Dev Ctries. 2014; 8: 676-679.
- Bayer AS, Bolger AF, Taubert KA, Wilson W, Steckelberg J, Karchmer AW, et al. Diagnosis and management of infective endocarditis and its complications. Circulation. 1998; 98: 2936-2948.
- Jalanko H. Congenital nephrotic syndrome. Pediatr Nephrol. 2009; 24: 2121-2128.
- Roy RR, Roy E, Rahman MH, Hossain MM. Serum immunoglobulin G, M and IgG:IgM ratio as predictors for outcome of childhood nephrotic syndrome. World J Pediatr. 2009; 5: 127-131.
- Holmberg C, Antikainen M, Rönnholm K, Ala Houhala M, Jalanko H. Management of congenital nephrotic syndrome of the Finnish type. Pediatr Nephrol. 1995; 9: 87-93.
- Silvestre JF, Betlloch MI. Cutaneous manifestations due to Pseudomonas infection. Int J Dermatol. 1999; 38: 419-431.
- Freeman JJ, Gadepalli SK, Siddiqui SM, Jarboe MD, Hirschl RB. Improving central line infection rates in the neonatal intensive care unit: Effect of hospital location, site of insertion, and implementation of catheterassociated bloodstream infection protocols. Pediatr Surg. 2015; 50: 860- 863.
- McDonald LW, Rhoads PS, Knapp AK. Bacterial endocarditis due to Pseudomonas aeruginosa; Report of a case. J Am Med Assoc. 1958; 167: 1490-1493.
- Harris HW, Umetsu D, Geha R, Harmon WE. Altered immunoglobulin status in congenital nephrotic syndrome. Clin Nephrol. 1986; 25: 308-313.