Case Report
A Newborn with Pulmonary Atresia and Antenatal Colchicine Exposure
Fırat Ergin1, Demet Terek1, Mehmet Yalaz1, Ertürk Levent2, Özge Altun Köroğlu1, Sibel Göksel3, Mete Akısü1, Ferda Özkınay4 and Nilgün Kültürsay1*
1Department of Pediatrics, Division of Neonatology, Ege University, Turkey
2Department of Pediatrics, Division of Cardiology, Ege University, Turkey
3Department of Pharmacology, Ege University, Turkey
4Department of Pediatrics, Division of Genetics, Ege University, Turkey
*Corresponding author: Nilgün Kültürsay, Ege University Children Hospital, Head and Division of Neonatology, 35100, Bornova, İzmir, Turkey
Published: 01 Mar, 2017
Cite this article as: Ergin F, Terek D, Yalaz M, Levent E,
Köroğlu ÖA, Göksel S, et al. A Newborn
with Pulmonary Atresia and Antenatal
Colchicine Exposure. Ann Clin Case
Rep. 2017; 2: 1284.
Abstract
Colchicine treatment improved female fertility and the outcome of pregnancy in Familial Mediterranean Fever patients by preventing the serosal adhesions and controlling the acute attacks. Limited number of human series does not show any proof for increased abortus, stillbirth or teratogenic effect of colchicine. Here we present a term newborn with pulmonary atresia whose mother was on colchicine during pregnancy. These two events may be coincidentally related; however, there may be a causal relationship that has not been reported in the literature yet. Fetal echocardiography is strongly recommended for women who are pregnant and taking colchicine.
Keywords: Colchicine; Teratogen; Newborn; Heart defect; Pulmonary atresia
Introduction
Colchicine treatment improved female fertility and the outcome of pregnancy in Familial
Mediterranean Fever patients by preventing the serosal adhesions and controlling the acute attacks.
Familial Mediterranean Fever (FMF) is a hereditary autoinflammatory disorder, characterized
by acute attacks of fever and serosal inflammation which is more frequent in Jewish, Armenian,
Turkish and Arabic populations [1]. Untreated or inadequately treated patients have the risk of
amyloidosis, which is an important cause of morbidity and mortality. Colchicine has been used in
the treatment of FMF since the 1970s and remains unrivalled in this respect [2].
It is necessary to control FMF attacks during pregnancy, because peritonitis may lead to early
contractions of the uterus and eventual abortions [3]. However, colchicine treatment improved
female fertility and the outcome of pregnancy by preventing the serosal adhesions and controlling
the acute attacks. Limited number of human series does not show any proof for increased abortus,
stillbirth, or teratogenic effect of colchicine. Therefore, the present opinion is that female patients
with FMF should continue taking the optimal dose of colchicine during their pregnancy and that
there is no need for amniocentesis during pregnancy [1,4]. Here we present a term newborn with
pulmonary atresia that may be a teratogenic effect of antenatal colchicine exposure.
Case Presentation
A 4110 gram, male infant with gestational age of 38 weeks, was delivered via cesarean section
from 31-year-old primiparous mother with FMF. The mother was on 1.5 mg/day colchicine
treatment. The infant’s Apgar scores were 9 and 10. Infant was in normal appearance and acyanotic.
His pulse oximetry oxygen saturation levels were around 90% at preductal and 88% at postductal
sites. A systolic ejection murmur was heard. He was admitted to neonatal intensive care unit due
to antenatal diagnosis of pulmonary stenosis (PS) on fetal echocardiography performed at 24th
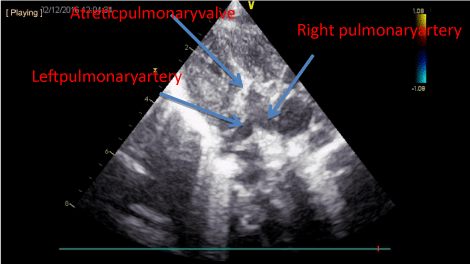
gestational week. Postnatal echocardiography revealed pulmonary atresia (PA) with no flow at
pulmonary valve, right ventricle was hypertrophic with a second-degree tricuspid insufficiency
(Figure 1). Pulmonary artery was filled via ductus arteriosus with a left to right shunt. Left ventricle
and left atrium size were normal.Immediate prostaglandin infusion was initiated and urgent thorax
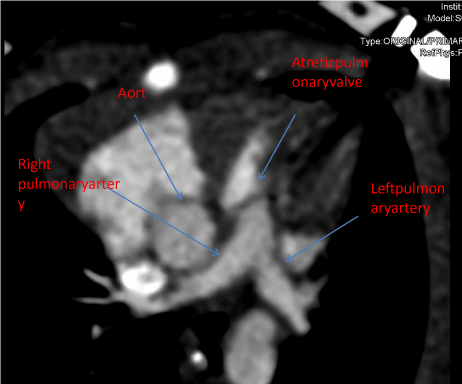
computed tomography angiogram confirmed an atretic pulmonary valve and anatrial septal defect
(Figure 2).
The initial complete blood count, biochemistry and acute phase reactant studies were normal.
The infant was operated on his third day of life. Since the pulmonary
valve was nearly totally atretic valvulotomy, valvuloplasty could not
be performed and a modified Blalock Taussig shunt was performed.
Some epileptiform movements were seen on the sixth day of life
and Levetiracetam therapy was started. Neurological examination,
Electroencephalography (EEG), and cranial Magnetic Resonance
Imaging (MRI) were performed. Low and immature activity for his
post-conceptional age was reported in EEG. MRI revealed a hyper
intense region at both parietal white matter which may be due
hypoxia related to his severe cardiac defect. As etiologic investigation
of cardiac defect, infant’s TORCH serology and FISH analysis for Di
George Syndrome and karyogram analysis resulted normal.
Figure 1
Figure 2
Discussion
We here report a newborn infant with isolated severe valvular PA
born to a mother with FMF treated with colchicine throughout her
pregnancy.
Pulmonary stenosis is a common congenital heart defect,
characterized by flow obstruction from right ventricle to pulmonary
arteries. It occurs in 0.6 to 0.8 per 1000 live births either isolated or
associated with some genetic syndromes [5]. It may also develop
due some perinatal infections. PS may be a component of the
syndromes such as Noonan syndrome, Alagille syndrome, Williams-
Beuren syndrome and Congenital rubella syndrome [1,5]. So far, the
relationship between FMF, colchicine treatment and PA have not
been reported.
In cases with critical PA, the severity of the right ventricular
outflow tract (RVOT) obstruction is life-threatening, because
of inadequate antegrade pulmonary blood flow and survival is
dependent on maintaining patency of the ductus arteriosus by the
administration of prostaglandin E1 (alprostadil) therapy. Our patient
had a severe PA which was diagnosed antenatally and urgently treated
with prostaglandin infusion followed by an MBT shunt deviating
some systemic flow to pulmonary arteries.
Increased infertility is seen, particularly in untreated women with
FMF, as a result of ovulatory insufficiency and peritoneal adhesions
(because of attacks or operations). At present, the gold standard
treatment for FMF is colchicine. The incidence of spontaneous
abortions before colchicine treatment is around 25–30% [2].
Development of peritoneal adhesions can be decreased and fertility
can be achieved, with colchicine treatment. Some studies have
reported that premature births and spontaneous abortions are also
increased in patients treated with colchicine [6].
There are some concerns about colchicine use in perinatal period.
Colchicine acts on the microtubular structure of cells and may affect
various cellular processes such as mitosis. Laboratory studies have
reported that chromosome aberrations due to the negative effect
of colchicine on mitosis [7]. Animal data shows that colchicine
and its derivative demecolcicine is teratogenic in mice and rabbits
in low doses and embryocidal in mice, rats and rabbits in higher
doses [8]. However, animal studies with colchicine did not result in
any chromosome aberrations. No congenital malformations were
reported in a small number of human fetuses exposed to colchicine
[9].
Colchicine may cause morphological abnormalities by induction
of vascular epithelial cell apoptosis via JNK activation [10]. Antenatal
colchicine exposure may be related to structural malformation in a
similar way. Cancer studies in adults also shows antivascular effects
by disruption of cytoskeletal structure [11].
Colchicine is in category C in FDA Reports and in category D in
New Zealand and Australia Reports. Demonstration of the passage
of colchicine through the placenta and case reports of trisomy in the
children of women using colchicine have caused serious concerns.
Analysis of large-scale monitoring data showed that no difference in
cytogenetic abnormalities was observed in patients taking colchicine
during pregnancy compared with those not taking colchicine [12].
The reported cases with trisomy 21 is thought to be coincidental
.
Therefore, cessation of colchicine should not be recommended
in conception and throughout pregnancy even in patients with
symptomatic remission. As amniocentesis is an invasive procedure
with a risk of miscarriage and infection, some investigators
have questioned the need for this procedure and reported that
amniocentesis was not required in patients taking colchicine during
pregnancy [13].
Colchicine may be excreted into breast milk. Studies have reported
that the drug reached the maximum level in breast milk 1-2 hours
after administration. On the other hand, when the amount passed
to the child was analyzed, it was observed to be only one-tenth of
the maternal dose [14]. Therefore colchicine is safe in breast feeding
mother, but mothers are recommended not to breastfeed within 0.5-2
hours of oral drug intake which is the peak time for serum drug levels.
In our case, maternal colchicine treatment and severe PS and
some hypoxic brain manifestations which are probably due to the
severity of PS in the offspring, may be coincidentally related; but it
may also show a rare causal relationship that was not reported in literature so far. Fetal echocardiography is strongly recommended for women who are pregnant and taking colchicine.
References
- Onen F. Familial Mediterranean fever. Rheumatol Int. 2006; 26: 489-496.
- Sarı İ, Birlik M, Kasifoğlu T. Familial Mediterranean fever: An updated review. Eur J Rheumatol. 2014; 1: 21-33.
- Ben-Chetrit E, Levy M. Reproductive system in familial Mediterranean fever: an overview. Ann Rheum Dis. 2003; 62: 916-919.
- Ehrenfeld M, Brzezinski A, Levy M, Eliakim M. Fertility and obstetric history in patients with familial Mediterranean fever on long-term colchicine therapy. Br J Obstet Gynaecol. 1987; 94: 1186-1191.
- Peng LF, Perry S. Pulmonic stenosis (PS) in neonates, infants, and children. 2016.
- Ofir D, Levy A, Wiznitzer A, Mazor M, Sheiner E. Familial Mediterranean fever during pregnancy: an independent risk factor for preterm delivery. Eur J Obstet Gynecol Reprod Biol. 2008; 141: 115-118.
- Hansteen IL. Colchicine and chromosome aberrations. Lancet. 1969; 2: 744-745.
- Shepard TH. Catalogue of teratogenic agents. 6th edn. Johns Hopkins University Press, Baltimore, USA. 1989; 164-166.
- Briggs G, Freeman RK, Taffe SJ. Drugs in lactation and pregnancy. 7th edn. Lippincott Williams and Wilkins, Philadelphia. 2005; 379-381.
- Hu YL, Li S, Shyy JY, Chien S. Sustained JNK activation induces endothelial apoptosis: studies with colchicine and shear stress. Am J Physiol. 1999; 277: H1593-H1599.
- Dalyot-Herman N, Delgado-Lopez F, Gewirtz DA, Gupton JT, Schwartz EL. Interference with endothelial cell function by JG-03-14, an agent that binds to the colchicine site on microtubules. Biochem Pharmacol. 2009; 78: 1167-1177.
- Diav-Citrin O, Shechtman S, Schwartz V, Avgil-Tsadok M, Finkel- Pekarsky V, Wajnberg R, et al. Pregnancy outcome after in utero exposure to colchicine. Am J Obstet Gynecol. 2010; 203: e1-e6.
- Ben-Chetrit E, Ben-Chetrit A, Berkun Y, Ben-Chetrit E. Pregnancy outcomes in women with familial Mediterranean fever receiving colchicine: is amniocentesis justified? Arthritis Care Res. 2010; 62: 143-148.
- Ben-Chetrit E, Scherrmann JM, Levy M. Colchicine in breast milk of patients with familial Mediterranean fever. Arthritis Rheum. 1996; 39: 1213-1217.