Case Report
A Case of Primary Retroperitoneal Serous Adenocarcinoma and Literature Review
Meng-meng Zhang, Xi-wa Zhao and Jian Zhao, Shan Kang*
Department of Obstetrics and Gynaecology, Hebei Medical University, China
*Corresponding author: Shan Kang, Department of Obstetrics and Gynaecology, Hebei Medical University, Fourth Hospital, Shijiazhuang, China
Published: 21 Feb, 2017
Cite this article as: Meng-meng Zhang, Xi-wa Zhao,
Zhao J, Kang S. A Case of
Primary Retroperitoneal Serous
Adenocarcinoma and Literature Review.
Ann Clin Case Rep. 2017; 2: 1277.
Abstract
Primary retroperitoneal serous adenocarcinoma (PRSA) is an extremely rare clinical entity with only seven cases previously reported. In this article, we described a 58-year-old woman with a solitary tumor of serous adenocarcinoma located in the right side of the Douglas Pouch arising from the retroperitoneum. Although several theories have been proposed, the exact origin of PRSA remains unclear. The most widely accepted theory is caelomic metaplasia. According to the histopathological results of the present case, the concept of caelomic metaplasia has been proved to a certain extent. As far as we know, this may be the first case which provides a direct and potent evidence for this theory and is conductive to explain the histogenesis of PRSA. The patient underwent a treatment modality combining complete surgical resection with platinum-based chemotherapy, which is extremely likely to contribute to an excellent clinical outcome, and we consider that such therapeutic strategy may be the preferred method for the treatment of PRSA.
Introduction
Primary retroperitoneal serous adenocarcinoma (PRSA) is an extremely rare malignancy, about which only seven cases previously reported as a result of a careful search in PubMed database from 1991 to December 2016 [1-7]. All of the reported patients were women ranging in age from 11 to 75 years. Because of the rarity, the histogenesis of PRSA remains unknown, the biological behavior, clinical manifestation and imaging finding of the tumors are various and the diagnostic criteria and therapeutic strategy of this entity are still not unified [2,5-7]. A solitary solid tumor in the Douglas pouch from the retroperitoneum has not been described in previous reports. Here we report a rare case of PRSA and review the relevant literatures.
Case Presentation
A 58-year-old postmenopausal woman, gravida2, parity1, first visited our Department in
July 2014 with a three-week history of alternate stool abnormality. She had no medical history of
abdominal surgery or lesion. A tumor 43*33*26 mm in size located behind the cervix was detected
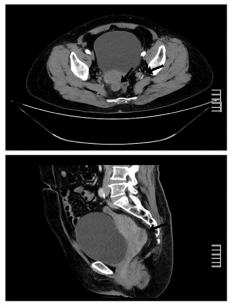
by ultrasound transvaginal examination. A pelvic computed tomography (CT) scan showed a
low-density solid neoformation with an irregular and enhanced margin which was adjacent to
the rectum in cul-de-sac and multiple enlarged lymph nodes in celiac and pelvic cavity (Figure
1). Gastrointestinal investigations, including gastrointestinal scopy and colonoscopy were normal.
Laboratory studies demonstrated elevated serum levels of CA-125, 3942.00 U/ml.
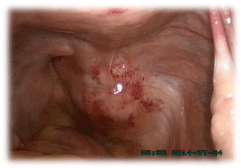
For further work-up, a laparoscopy was performed. We detected a solitary solid-appearing
mass measuring 4 cm in diameter approximately in the right side of the Douglas Pouch, which
was located in the retroperitoneum and squeezed the rectum to the left (Figure 2). The size
and appearance of uterus, bilateral fallopian tubes, ovaries, peritoneum, omentum majus and
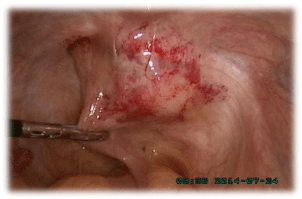
mesentery were normal. Considering that the tumor was closely attached to the rectum wall
(Figure 3), a surgeon was invited to consult to exclude rectal neoplasm. A complete resection
of the retroperitoneal tumor was conducted, and the histopathologic examination of a frozen
section showed low-differentiated adenocarcinoma. Subsequently, a total hysterectomy, bilateral
salpingo-oophorectomy, omentectomy, pelvic and paraaortic lymphadenectomy and biopsy of the
peritoneum were performed.
Cytological analysis of peritoneal washing liquid showed no abnormalities. Postoperative
histopathology examination of the specimen showed a poorly-differentiated adenocarcinoma
involving the tumor. There were no microscopic invasion of the uterus, ovaries and tubes, omentum
and lymph nodes. Immunohistochemistry of the mass pathology revealed positive findings for CK, CA124, and 70% positive for Ki-67, partly positive for MC and negative
findings for CEA, Vim, Calret and CDX2. The pathology consultation
results in Peking University Third Hospital demonstrated a
peritoneum original high-grade serous adenocarcinoma; meanwhile,
a small amount of benign mucosal epithelium was seen beside the
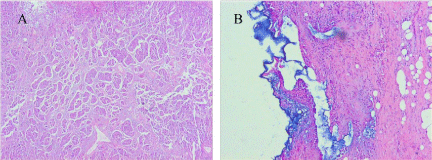
tumor tissue, which showing morphological differentiation towards to
the epithelium of the fallopian tube (Figure 4A and B). Consequently,
a histological diagnosis of PRSA was made, and defined at stage II
according to the 2006 FIGO classification.
The patient was subsequently treated with platinum-based
chemotherapy, a combination of paclitaxel and carboplatin (TC
therapy, 3-week intervals). After a total of seven courses of TC
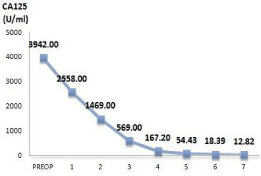
therapy, there was a significant reduction on the level of serum CA125
from 2558.00 U/ml to 12.82 U/ml (Figure 5) and CT scan showed
no evaluable disease. 21 months after the surgery, positron emission
tomographic (PET) scan revealed a retroperitoneal nodule in pelvic
cavity and a suspicious bone metastasis in sternum. The patient was
once again treated with chemotherapy, which was effective as before.
When last seen on January 2017, the patient has been alive with stable
disease for 30 months since her surgery and the follow-up is still continuing.
Figure 1
Figure 1
Pelvic computed tomography scan showing a solid tumor in cul-desac
and enlarged lymph nodes in pelvic cavity (arrows).
Figure 2
Figure 2
A solitary solid-appearing tumor in the right side of the Douglas
Pouch from the retroperitoneum.
Figure 3
Figure 4
Figure 4
(A) Histological findings of the retroperitoneal tumor, showing solid
growth of atypical epithelial cells with pleomorphic nuclei and frequent mitotic
figures; (B) a small number of benign mucosal epithelium demonstrating tubal
differentiation in morphology beside the tumor tissue (HE staining ×100).
Figure 5
Figure 5
Change of serum CA-125 levels along the treatment.
Note: PREOP: Pre-operation 1: first course of chemotherapy
Discussion
PRSA is an extremely rare malignancy disorder with only seven
cases previously reported [1-7]. To explain the exact histogenesis of
the PRSA, several theories have been postulated including coelomic
metaplasia, extraovarian endometriosis, ectopic ovarian tissue,
supernumerary ovaries, teratoma and enterogenic cyst, currently the
most widely accepted one is caelomic metaplasia. The peritoneum has
been defined as the secondary Müllerian system for similar potential
to differentiate into various types of epithelium derived from the
Müllerian tube: endometrium, endocervix, and fallopian tube [8,9].
It is believed that the epithelial metaplasia of peritoneal mesothelium
is one of the originations of the extragenital Müllerian neoplasms.
The metaplastic epithelium has a tumorigenic potential, resulting
in the formation of a variety of tumors including serous, mucinous,
endometrioid and clear cell tumors. Singh et al. [10] point out that
there is sufficient and clear evidence to prove that fallopian tube is the origination of non-uterine high grade serous carcinoma (HGSC).
Gruessner et al. [11] have reported a case in which a potential link
between endosalpingiosis (ES) and the development of pelvic serous
carcinoma in woman has been observed. Therefore, it is conceivable
that epithelial retroperitoneal tumors may have initially been
metaplastic peritoneal mesothelium.
However, currently there is no case directly confirms that
PRSA originates in the metaplasia of peritoneal mesothelium. The
pathological consultation result of the present case clearly mentioned
that a small number of the benign mucosal epithelium demonstrating
tubal differentiation in morphology can be detected beside the tumor
tissue, suggesting that the primary retroperitoneal tumor may come
from metaplasia of peritoneal mesothelial cells. It confirms that the
peritoneum is the matrix where benign and malignant tumors of
secondary Müllerian epithelium occur [12]. To our best knowledge,
this may be the first case which provides a direct and potent evidence
for the theory of caelomic metaplasia and contributes to clarify the
histogenesis of PRSA.
It is believed that the biological behavior of PRSA is similar to
epithelial ovarian cancer. According to the reported cases, the main
clinical symptoms of PRSA are asymptomatic abdominal and pelvic
mass, abdominal pain, vomiting, anorexia, abdominal distension and
weight loss, resembling those of ovarian serous adenocarcinoma.
Despite there is no basic standard of treatment for the disease at
present, patients were treated in accordance with the treatment
fashion of epithelial ovarian cancer. Among the seven reported
cases, four patients underwent complete tumor resection and partial
resection of ambient involved tissue. Two of the four patients
received chemotherapy, one was alive with disease for 32 months [6]
and the other survived 6 months with no clinical evidence of tumor
recurrence [7]; the rest two patients did not receive further therapy
following surgery, and survived for 24 months [3] and 7 months
[5] without recurrence, respectively. Ulbright et al. [1] reported a
case of a retroperitoneal neoplasm in an 11-year-old girl. The girl
underwent a partial resection of tumor and chemotherapy and was
alive with no evidence of disease for 10 months. Fujiwara et al. [4]
described a female with a retroperitoneal tumor who was only treated
with chemotherapy. She died of disease 24 months after initial
presentation. In addition, the case reported by Caruncho et al. [2] did
not inform patient's final outcome.
In the present case, patient underwent a radical operation
followed by adequate platinum-based chemotherapy, and obtained
an excellent clinical outcome. The patient was alive with no evidence
of disease for 21 months. Given chemotherapy after recurrence, she
survived up to now. The outcome of the patient demonstrated the
potential effectiveness and feasibility of this treatment pattern. In the
literature, it has been emphasized that patients with positive surgical
margins, tumor infiltration of adjacent organs or loco-regional
lymph node involvement should be treated with chemotherapy [3,5].
Although more cases are needed, we believe that the therapy modality
combining a radical operation with platinum-based chemotherapy
may be the top candidate for treatment of PRSA. Meanwhile, we must
pay attention to the adverse reactions of chemotherapy. Yonehara
et al. [13] have described a woman with primary serous papillary
carcinoma (PSPC), after first chemotherapy course, pericardial
effusion occurred and a pericardiectomy was performed to prevent
cardiac failure.
Conclusion
In conclusion, we report an unusual case of a 58-year-old woman with primary retroperitoneal serous adenocarcinoma, and the postoperative pathology provides powerful evidence for the theory of caelomic metaplasia. Although more cases are needed, we emphasize that radical surgery combined with platinum-based chemotherapy may be the preferred method for treatment of PRSA.
Acknowledgement
We acknowledge Wei Zhao, Li-xian Wang and Zhao-zi Pang, who facilitated our work in this case, report. We thank Department of Pathology for their excellent technical assistances.
References
- Ulbright TM, Morley DJ, Roth LM, Berkow RL. Papillary serous carcinoma of the retroperitoneum. Am J Clin Pathol. 1983; 79: 633-637.
- Caruncho M, Pombo F, Arnal-Monreal F. Primary retroperitoneal serous cystadenocarcinoma of “ovarian type”: US and CT findings. Eur J Radiol. 1993; 17:115-116.
- Kurosaki Y, Kuramoto K. Case report: serous cystadenocarcinoma of the retroperitoneum: CT and sonographic appearance. Clin Radiol. 1998; 53: 916-918.
- Fujiwara K, Oda T, Suzuki S, Kohno I, Hirokawa M. Primary serous adenocarcinoma of the retroperitoneum with a response of platinumbased chemotherapy: a case report. Int J Gynecol. Cancer 1999; 9: 170-172.
- Kaku M, Ohara N, Seima Y, Imanishi K, Tomura N, Kobayashi A, et al. A primary retroperitoneal serous cystadenocarcinoma with clinically aggressive behavior. Arch Gynecol Obstet. 2004; 270: 302-306.
- Iura A, Sasajima Y, Katsumata N, Kasamatsu T. Serous adenocarcinoma of the retroperitoneum, as a type of multifocal mullerian carcinoma. Int J Clin Oncol. 2009; 14: 254-257.
- Arichi N, Yasumoto H, Mitsui Y, Hiraoka T, Honda S, Shiina H, et al. A case of primary retroperitoneal serous adenocarcinoma. Int J Urol. 2011; 18: 844-846.
- Lauchlan SC. The secondary müllerian system. Obstet Gynecol Surv. 1972; 27: 133-146.
- Berretta R, Patrelli TS, Faioli R, Mautone D, Modena AB, Gizzo S, et al. Secondary müllerian system: an atypical case of tumor originating from vestigial müllerian cells embedded in the peritoneum. Clin Genitourin Cancer. 2013; 11: 365-369.
- Singh N, Gilks CB, Wilkinson N, McCluggage WG. The secondary müllerian system, field effect, BRCA, and tubal fimbria: our evolving understanding of the origin of tubo-ovarian high-grade serous carcinoma and why assignment of primary site matters. Pathology. 2015; 47: 423-431.
- Christine Gruessner, Angelika Gruessner, Katherine Glaser, Nisreen AbuShahin, Cynthia Laughren, Wenxin Zheng, et al. Biomarkers and endosalpingiosis in the ovarian and tubal microenvironment of women at high-risk for pelvic serous carcinoma. Am J Cancer Res. 2014; 4: 61-72.
- Lauchlan SC. The secondary müllerian system revisited. Int J Gynecol. Pathol. 1994; 13: 73-79.
- Yonehara T, Yamaguchi T, Azuma M, Minobe S, Sakuragi N. A case of primary serous papillary carcinoma with unusual clinical presentation: distant lympho nodes metastasis without peritoneal dissemination. Arch Gynecol Obstet. 2008; 278: 579-583.