Case Report
Dural Marginal Zone Lymphoma Presented as Brain Herniation: A Case Report
Huda A Al-Jadiry2, Ahmed Q Hasan1*, John Heyman2 and Olajumoke O Oladipo3
1Department of Emergency Medicine, Louisiana State University, USA
2Department of Radiology, University of Texas Medical Branch, USA
3Department of Pathology, University of Texas Medical Branch, USA
*Corresponding author: Ahmed Q Hasan, The University of Texas Medical Branch, 301 University Boulevard, Galveston, Texas, USA
Published: 12 Jan, 2017
Cite this article as: Hasan AQ, Al-Jadiry HA, Heyman
J, Oladipo OO. Dural Marginal Zone
Lymphoma Presented as Brain
Herniation: A Case Report. Ann Clin
Case Rep. 2017; 2: 1232.
Abstract
Spontaneous delayed rupture of the extensor pollicis longus (EPL) is a rare complication following distal radius fracture. Bilateral EPL rupture following bilateral distal radius fractures is even rarer. A case report of bilateral EPL rupture following bilateral non-displaced distal radius fractures is presented along with a review of the literature regarding pathophysiology, treatment options, and technical surgical variations.
Keywords: Primary dural lymphoma (PDL); Primary central nervous system lymphoma (PCNSL); Marginal zone lymphoma (MZL)
Case Presentation
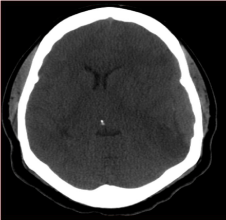
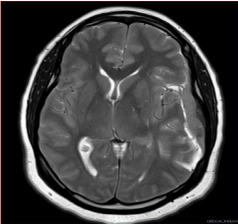
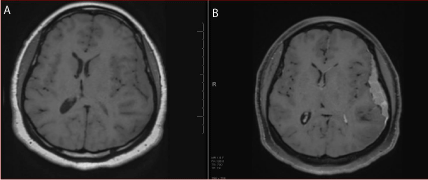
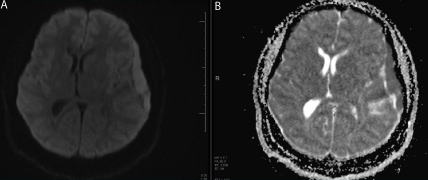
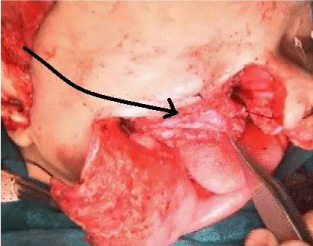
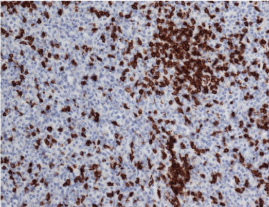
A 38 year old, African American female presented to the primary care physician with a chief complaint of new onset headache and blurred vision of three months duration. The headache had worsened during the past two weeks. She also had visual changes, described as a curtain going down with flashes of light, and reported attacks of gait imbalance and left facial droop. The patient was referred to Ophthalmology clinic for further assessment, which revealed bilateral grade III papilledema. The Ophthalmologist referred the patient to the emergency department for further neurological evaluation and brain MRI. Initial non-contrast brain CT scan showed a left crescentshaped slightly hyper dense lesion causing rightward midline shift resulting in subfalcine and uncal herniation which was interpreted as a subacute subdural hematoma (Figure1). Neurosurgery was consulted and Brain MRI was performed which showed a dural based lobulated T2WI isointense mass with trapped CSF (Figure 2) homogenous avid post contrast enhancement (Figure 3 A & B), and restricted diffusion (Figure 4 A & B) There were no associated calvarial bony changes or adjacent parenchymal vasogenic edema. The differential diagnosis based on MRI was atypical meningioma, hemangiopericytoma or Dural metastasis. Patient underwent craniotomy and resection of the mass and histopathology revealed diffuse lymphoid infiltrate with regressed germinal centers of varying sizes (Figure 5) with predominant CD20 B cells and fewCD3 positive T cells (Figure 6). CD20 highlights the B cells within and between the follicles (Figure 7). These findings are consistent with marginal zone lymphoma and the presence of germinal centers suggests Nodal type (Figure 7). The patient underwent CT neck, chest, abdomen and pelvis to evaluate for systemic disease, which was negative. Extensive laboratory work up including CSF analysis, viral screen and bone marrow biopsy were also all negative for systemic lymphoma.
Figure 1
Figure 1
Axial non contrsted Brain CT Scan: Left elliptical shaped isodense
extraxial lesion( Arrow).
Figure 2
Figure 3
Figure 3
(A) 3T Brain MRI Axial T1 WI without Gadolinium: Isointense left
convexity extra axial lesion ( Arrow). (B) 3T Brain MRI Axial T1 WI post
Gadolinium: Homogenous avidly enhancing left convexity extra axial lesion.
Discussion
Dural masses result from variable tumors and disorders and maybe either primary or secondary.
The most common primary dural neoplasm is meningioma followed by hemangiopericytma.
Secondary neoplasms of the dura result from intracranial masses or systematic malignancy [1].
PDL is a rare subtype of PCNSL. Primary CNS lymphoma is neoplastic process of leptomeninges,
brain, spinal cord, ventricles and eyes without evidence of nodal or extra nodal disease outside CNS.
Secondary CNS involvement by lymphoma is much more common [2].
PCNSL can occur in both immune compromised and immune competent patients and represents
approximately 2.7% of all primary and secondary CNS malignancies. PDL accounts for less than 1% of all CNS lymphomas [3,4]. Bhagavathi et al [5]. reported an
increased incidence of PCNSL over the past three decades. It is more
common in middle-aged females with a male-to-female ratio of 1:2
to1:7 and a median age of 56 years. It tends to occur in a younger age
group in immune compromised patients [3,4]. The etiological factors
predisposing to PCNSL are not completely understood; the only
known risk factor is congenital or acquired immunodeficiency [6].
PCNSL is subdivided based on histopathological findings.
Diffuse large B-cell lymphoma, accounting for 90%, is most common
followed by T-cell lymphoma and then Marginal zone lymphoma [7].
PCNSL is solitary in 65% and multifocal in 35% of cases [4].
Lymphoma that arises from the dura is low-grade B-cell marginal
zone lymphoma. MZL is a non-Hodgkin lymphoma, which most
commonly occurs in the gastrointestinal tract where it called “mucosa
associated lymphoid tissue (MALT). It is treated differently and has a different prognosis than other types of PCNSL [8].
The pathogenesis of the PDL is not totally understood, as the
Dura is free of any lymphoid tissue [6]. Few presumptive theories
were suggested (a) Dural lymphoma is the Consequence of seeding
from an undiagnosed systemic MALT lymphoma; (b) meningothelial
cells at the arachnoid membrane and Dural venous sinuses are
embryologically similar to epithelial cells at other sites in which
MALT lymphomas arise, and (c) Attraction of the polyclonal
lymphocytes by inflammatory conditions of the Dura can give rise to
MALT lymphoma [9,10].
Dural lymphomas appear as hyper- or isoattenuated masses
on CT due to high cellularity and almost all demonstrate iodinated
contrast enhancement. Negative CT scan does not rule out PCNSL
[11,12]. On MRI, PDL shows iso- to hypointensity on T2WIand
vivid post Gadolinium enhancement sometimes with heterogeneity
[13]. It is easy to mistake for meningioma or subdural hematoma as
these both show hyperdensity on CT scan [13]. MR imaging utilizing DWI perfusion as lymphoma demonstrates diffusion restriction due
to high cellularity and spectroscopy manifested by lipid peak and
increased Cho/Cr ratio might have potential to aid the differentiation
between lymphoma and other dural tumors [14].
PDL has a favorable prognosis compared to parenchymal PCNSL
subtypes or Dural metastasis of systemic lymphoma. Due to the
rarity of the disease, yet there is no standardized treatment plan;
however, disease limited to a single site has a good response to local
treatment such as surgery or focal radiation [15]. In conclusion,
MZL is a rare tumor with vague nature of the clinical and the
radiological characteristics. The author’s main objective in this case
is to raise the level of awareness and preparedness of the radiologists
about this dural tumor that warrants further workup to plan proper
management for such cases.
The uniqueness of this case report comes from the unusual
presentation, which in this case was brain herniation and was initially
interpreted as subdural hematoma despite of the absence of trauma
and the young age of the patient. This case also demonstrates the
importance of proper communication and cooperation between
specialties and its critical role in providing high-quality service.
Finally as radiologist we owe it to our patients and ourselves to
practice evidence-based medicine to support our clinical decisions
which ultimately will result in a better patient`s care.
Figure 4
Figure 4
(A) 3T Brain MRI DWI sequence. (B) ADC Map Sequence.
Restricted diffusion pattern seen in the left convexity extra axial lesion (
Arrows ).
Figure 5
Figure 5
Diffuse Lymphoid infiltrate with regressed germinal centers of
varying sizes (Arrow) (x4 magnification).
Figure 6
References
- N.L.N Murthy, P Madhuri, Dr. Ashok, Dr.S.Srinivas. Primary dural lymphoma: case report. IOSR JDMS 2015; 18:41-43.
- Haldorsen IS, Espeland A, Larsen JL, Mella O. Diagnostic delay in primary central nervous system lymphoma. Acta Oncol. 2005; 44: 728-734.
- Central Brain Tumor Registry of the United States (2002–2003) Primary brain tumors in the United States 1995–1999: statistical report. CBTRUS, Chicago, IL.
- JL Rubenstein, N K Gupta, G. N. Mannis, A. K. Lamarre, P. Treseler, “How I treat CNS lymphomas,” Blood. 2013; 122: 2318-2330.
- Sharathkumar Bhagavathi, Timothy C Greiner, Syed A Kazmi, Kai Fu, Warren G Sanger, and Wing C Chan. Extranodal marginal zone lymphoma of the dura mater with IgH/MALT1 translocation and review of literature. J Hematop. 2008; 1: 131-137.
- Fabio M Iwamoto, Lauren E Abrey. Primary Dural lymphoma: A review. Neurosurg Focus. 2006; 21:E5.
- Monabati A, Rakei SM, Kumar P, Taghipoor M, Rahimi A. Primary Burkitt lymphoma of the brain in an immunocompetent patient: case report. J Neurosurg. 2002; 96: 1127-1129.
- Kleihues P, Cavenee WK (2000) World Health Organization classification of tumors: pathology and genetics: tumors of the nervous system. IARC, Lyon.
- Ferguson SD, Musleh W, Gurbuxani S, Shazadeh SF, Lesniak MS. Intracranial mucosa-associated lymphoid tissue (MALT) lymphoma. J Clin Neurosci. 2010; 17: 666–669.
- Kumar S, Kumar D, Kaldjian EP, Bauserman S, Raffeld M, Jaffe ES. Primary low-grade B-cell lym- phoma of the dura: a mucosa associated lymphoid tissue–type lymphoma. Am J Surg Pathol. 1997; 21: 81-87.
- Haldorsen IS, Kråkenes J, Krossnes BK, Mella O, Espeland A. CT and MR imaging features of primary central nervous system lymphoma in Norway, 1989-2003. AJNR Am J Neuroradiol. 2009; 30: 744-751.
- Koeller KK, Smirniotopoulos JG, Jones RV. Primary central nervous system lymphoma: radiologic-pathologic correlation. Radiographics. 1997; 17: 1497-1526.
- Gocmen S, Gamsizkan M, Onguru O, Sefali M, Erdogan E. Primary dural lymphoma mimicking a subdural hematoma. J Clin Neurosci. 2010; 17: 380-382.
- IS Haldorsen A, Espeland E, M. Larsson. Primary Dural lymphoma: Characteristic findings on traditional and advances imaging. AJNR Am J Neuroradiol. 2011; 32: 984-992.
- Thieblemont C, Bastion Y, Berger F, Rieux C, Salles G, Dumontet C, et al. Mucosa-associated lymphoid tissue gastroin- testinal and nongastrointestinal lymphoma behavior: analysis of 108 patients. J Clin Oncol. 1997; 15:1624-1630.