Case Report
A Case of Nasal Squamous Cell Carcinoma with the Clinical Symptom of Recurrent Epistaxis in a Case of Nasal Squamous Cell Carcinoma
Cheng-Ku Tsai1* and Hung-Pin Wu1,2
1Department of Otolaryngology, TaichungTzu Chi Hospital, China
2Department of Medicine, HualienTzu Chi University, China
*Corresponding author: Cheng-Ku Tsai, Department of Otolaryngology, Taichung Chi Hospital, No.11812F-6, Yuk Tak Road, North District, Taichung City, Taiwan, China
Published: 26 Dec, 2016
Cite this article as: Tsai C-K, Wu H-P. A Case of Nasal
Squamous Cell Carcinoma with the
Clinical Symptom of Recurrent Epistaxis
in a Case of Nasal Squamous Cell
Carcinoma. Ann Clin Case Rep. 2016;
1: 1223.
Abstract
Malignant tumors in the nasal cavity and the paranasal sinus account for 1% of all cancers in the body. The majority of malignant tumors in the nasal cavity and the paranasal sinus are squamous cell carcinoma, accounting for approximately 60% of cancers in these locations. In August 2014, an 80-year-old woman sought treatment at the Department of Otolaryngology (Ear, Nose & Throat), Taichung Tzu Chi Hospital, with the main complaint of a 40-year history of recurrent Epistaxis in the right nasal cavity. The endoscopic examination revealed bleeding tumors in the right nasal cavity. The computed tomography examination revealed that the right nasal cavity and the right paranasal sinus were filled with homogeneous tumors. The patient underwent an endoscopic resection of the tumors in the paranasal sinus in October 2014. The pathological examination confirmed a diagnosis of squamous cell carcinoma. After explaining the pathological result to the patient's family, follow-up radiotherapy and chemotherapy were recommended for the patient, but the proposed treatment was rejected by the family members because the patient was old and weak. Squamous cell carcinomas in the nasal cavity and the paranasal sinus are rare; therefore, this case is report edhere. This case is unique because the patient was older (80 years old) and had a long history of recurrent Epistaxis (40 years). The patient had never sought treatment for this disease at any hospital or clinic and had never received any related examination, treatment, or surgery. This case was unique because it allowed for the observation of the original features of nasal malignant tumors after 40 years of growth.
Keywords: Squamous cell carcinoma; Epistaxis; Paranasal sinus; Homogeneous
Introduction
The nasal cavity the and paranasal sinus are closely related. They not only are in close proximity
anatomically but also contain similar epithelial tissue. The paranasal sinus has a natural ostium that
connect stothenasal cavity. Therefore, malignant tumors that occur in the nose can easily directly
invade the adjacent anatomical structures and continuously increase the extent of the disease. In
the case reported in this study, the nasal cavity and the paranasal sinus contained a large amount of
tumor tissue.
Malignant tumors in the nasal cavity and paranasal sinus account for1% of all cancers in the
body, and the incidence of these cancers in descending order is as follows: squamous cell carcinoma,
adenoid cystic carcinoma, and adenocarcinoma.The main symptoms include nasal congestion,
nasal bleeding, facial swelling, facial pain, and possibly headache, diplopia, or proptosis if the tumor
involves the skull base or the orbit. The early symptoms are not obvious and are often ignored by
the patient, leading to a later diagnosis, which results in delayed medical treatment. For example, in
the present case, because the recurrent nose bleeding was not severe, the patient had not sought any
related medical treatment for 40 years. At the first visit, the right nasal cavity and the right paranasal
sinus contained a large number of tumors. CT scan reveal that the tumor invades some surrounding
structures and erodes bone (medial wall of maxillary sinus, skull base, nasal septum).
Case Presentation
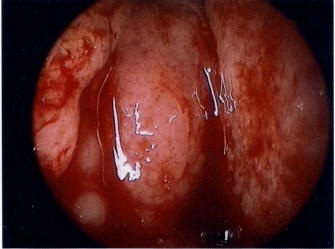
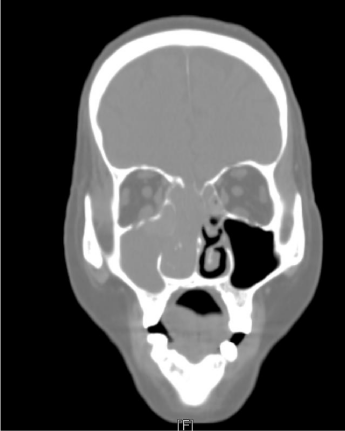
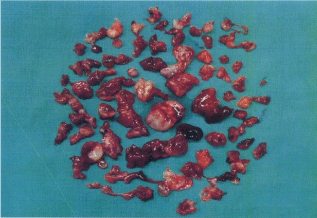
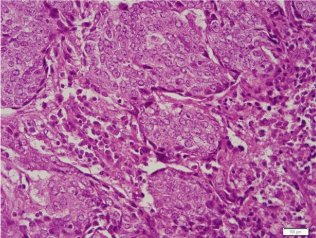
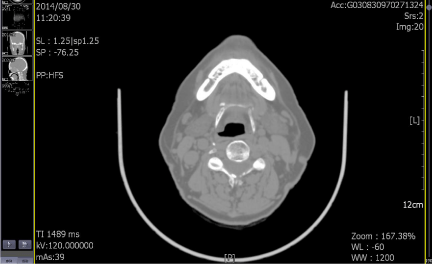
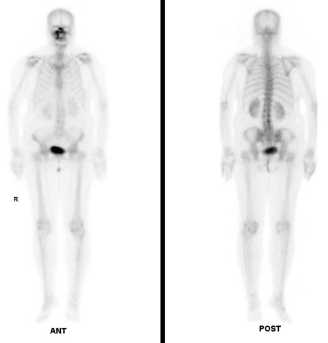
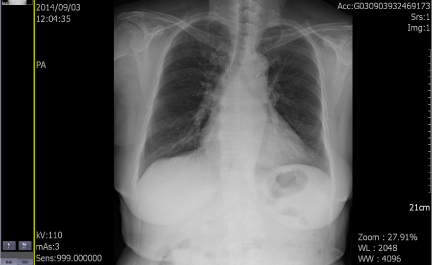
In August 2014, an 80-year-old female patient sought treatment for the first time at the Department of Otolaryngology (Ear, Nose & Throat) of our hospital, with the main complaint of a 40-year history of recurrent epistaxis in the right nasal cavity. She had no history official trauma, had never been in any hospital or clinic for related treatment, and had never received any related examination, treatment, or surgery. On the day of her initial visit, an endoscopic examination revealed bleeding tumors in the right nasal cavity (Figure 1), and a computed tomography examination revealed that the right nasal cavity and right paranasal sinus contained many homogeneous low-density tumors (Figure 2). CT scan reveal that the tumor invades some surrounding structures and erodes bone (medial wall of maxillary sinus, skull base, nasal septum). In October 2014, the patient underwent an endoscopic resection of the tumors in the paranasal sinus with general anesthesia, and many neoplastic pieces tumors were removed (Figure 3). The results of the pathological examination indicated squamous cell carcinoma, and increased nuclei and hyper chromatic chromatin were observed in the pathological microscopic sections (Figure 4). Neck CT Figure 5 reveal no cervical lymph node metastasis (No). Skeletal scan Figure 6 and chest X-ray Figure 7 show no distant metastasis (Mo). The tumor tissue had extended into the nasal cavity, maxillary sinus, frontal sinus, and ethmoid sinus, indicating a clinicalstageofcT4aNoMo. Because the family of the patient refused subsequent radiotherapy and chemotherapy, follow-up evaluations every six months were recommended.
Figure 1
Figure 2
Figure 2
The right nasal cavity and the right paranasal sinuses contained
many homogeneous low-density tumors.
Figure 3
Figure 4
Figure 4
Increased nuclei and hyper chromatic chromatin were observed in
the pathological microscopic sections.
Figure 5
Figure 6
Figure 7
Figure 8
Discussion
Malignant tumors of the nasal cavity and paranasal sinus account
for 1% of all cancers in the body and 5% of head and neck cancers.
The most common histological type is squamous cell carcinoma,
and the most common site is the maxillary sinuses [1]. The lesions
of malignant tumors produce histological components in areas of
the nasal cavity and the paranasal sinuses such as the Schneiderian
mucosa, salivary glands, neural tissue, and lymphatics. The locations
of malignant tumors in descending order of incidence include the
maxillary sinus (60%), the nasal cavity (20%), the ethmoid sinus (5%),
and the frontal and sphenoid sinuses (3%) [2]. Malignant tumors in
the nasal cavity and paranasal sinus are more likely to occur in men,
with a male to female ratio of 2.26:1. Racially, these tumors occur more
frequently in Caucasians. Studies have confirmed that the occurrence
of malignant tumors in the nasal cavity and paranasal sinusis related
to Epstein-Barr virus [3], and the development of squamous cell
carcinoma is related to human papilloma virus [4].
Malignant tumors in the nasal cavity and paranasal sinus may
not have any symptoms. Symptoms may be observed only when
the tumor has invaded the adjacent organs. The common clinical
symptoms include nasal congestion, nasal bleeding, palate mucosal
ulceration, loose or lost teeth, facial swelling, facial pain, diplopia,
and proptosis. The symptoms of malignant tumors in the nasal cavity
and paranasal sinus are not obvious; therefore, early diagnosis is
difficult. The disease is usually in an advanced stage at the time of
diagnosis, with a five-year survival rate of 30%-40%. Similar to other
malignancies, the clinical manifestations of malignant tumors in the
nasal cavity and paranasal sinus include (1) regional symptoms such
as neck lumps, diplopia, and epiphora; (2) local symptoms such as nasal obstruction and epistaxis; and (3) distant metastasis of the bone
[5]. The survival rate of patients with distant metastasis the lowest of
all cases. Dulguerov et al. found that the survival rate of the patients
in the T4 stage was the lowest of all cases [3].
For malignant tumors in the nasal cavity and paranasal sinus,
localized lymph node metastasis is more likely to occur in T2 tumors
than inT3 and T4 tumors [6] because T2 tumors invade the floor of
the maxillary sinus. A T2 tumor may involve in the following areas:
(1) the hard palate mucosa, (2) the upper gingiva mucosa, and (3) the
inferior nasal cavity mucosa. These three structures contain a dense,
rich lymphatic network, while the mucosa of the paranasal sinus lacks
of this feature; in terms of the incidence of lymph node metastasis, the
behavior of T2 tumors is more similar to that of oral cancer than that
of paranasal cancer. If the tumor tissue has invaded key structures
(the skull base, dura mater, brain, orbit, cavernous sinus, and infratemporal
fossa), the survival rate will be significantly reduced [7].
Treatment mainly consists of surgery combined with radiotherapy
and chemotherapy. The so-called tri modality therapy consists of
the following three methods [8]: (1) partial maxillectomy, (2) intraarterial
chemotherapy, and (3) radiotherapy. The intra-arterial
chemo therapy regimen includes cisplatin (100 mg/m2, four times
weekly), and the median irradiation dose of radiotherapy is 70Gy [9].
For patients with an advanced tumor stage (T3orT4), most scholars
recommended prophylactic radiotherapy (50Gy) on the non-meta
static cervical lymph nodes [7]. Except for lymphoma, radiotherapy
and chemotherapy have shown no direct efficacy on malignant
tumors [5]. The role of radiotherapy is to control the "regional
symptoms "and "local symptoms," and the role of chemotherapy is to
reduce the "tumor growth, "i.e., radiotherapy and chemotherapy are
clinically performed as adjuvant therapies for surgery.
For early stage tumors (stage I and stage II), radical resection is
the best surgical approach; for advanced stage tumors (stage III and
stage IV), adjuvant postoperative radiotherapy and chemotherapy
are necessary for a better prognosis. Endoscopic surgery is also an
alternative approach for early stage tumors. Many studies have
reported than endoscopic surgical treatment achieves excellent
efficacy for treatment of early stage malignant tumors in the nasal
cavity and paranasal sinus [10]. The advantages of endoscopic surgery
include (1) a small incision, less bleeding, and a reduced chance of post
operative infection; (2) no surgical scar on the face; and (3) a shorter
hospitalization period. Although the endoscopic surgery approach
has obvious advantages, it is not recommended for the following
cases: (1) the lesion involves the frontal sinus, (2) the lesion involves
the bone structure of the maxillary sinus, (3) the ulceration at the
bottom of the nasal cavity has extended to the upper jaw, and (4) the
lesion has invaded the orbit and skull base. The boundary of the tumor
should be assessed before the surgery. Computed tomography and
magnetic resonance imaging are the routine imaging examinations
used for these cases.
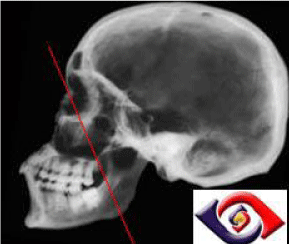
The straight line from the medial canthus of the eye to the angle
of the mandible is called Ohngren's line [2] (Figure 8). This line
divides the paranasal sinuses into anterior-inferior (infrastructure)
and superior-posterior (supra structure) sections. A carcinoma in
the infrastructure has a better prognosis, while a carcinoma in the
supra structure has a worse prognosis because the supra structure
carcinoma could extend into the eye orbit, skull base, and pterygoid
and infra-temporal fossae. Patients with early stage tumors may have
no symptoms, and advanced stage tumors could invade the bone structures of the orbit and skull base, leading to diplopia, proptosis,
and cerebrospinal fluid rhinorrhea.
The prognostic factors that affect local recurrence and distant
metastasis include (1) an advanced disease stage, (2) a non-surgical
treatment, (3) an aggressive histological types, (4) an intra-cranial
extension, and (5) a cervical lymph node metastasis; of which, cervical
lymph node metastasis is the most important prognostic factor.
According to the clinical statistics, cervical lymph node metastasis
occurs in 7% to 15% of patients.
References
- Homma A, Hayashi R, Matsuura K, Kato K, Kawabata K, Monden N, et al. Lymph Node Metastasis in T4 Maxillary Siuns Squamous Cell Carcinoma: Incidence and Treatment Outcome. Ann Surg Oncol. 2014; 21: 1706-1710.
- Kazi M, Awan S, Junaid M, Qadeer S, Hassan NH. Management of Sinonasal Tumors: Prognostic Factors and Outcomes: A 10 Year Experience at a Tertiary Care Hospital. Indian J Otolaryngol Head Neck Surg. 2013; 65: 155-159.
- Sanghvi S, Khan MN, Patel NR, Yeldandi S, Baredes S, Eloy JA. Epidemiology of Sinonasal Squamous Cell Carcinoma: A Comprehensive Analysis of 4994 Patients. Laryngoscope. 2014; 124: 76-83.
- Larque AB, Hakim S, Ordi J, Nadal A, Diaz A, del Pino M, et al. High-risk Human Papillomavirus is Transcriptionally Active in a Subset of Sinonasal Squamous Cell Carcinoma. Modern Pathology. 2014; 27: 343-351.
- Richard J HA, Dustin M DA. Sinonasal malignancies. American Journal of Rhinology&Allergy. 2013; 27: 35-38.
- Takes RP, Ferlito A, Silver CE, Rinaldo A, Medina JE, Robbins KT, et al. The Controversy in the Management of the No Neck for Squamous Cell Carcinoma of the Maxillary Sinus. Eur Arch Otorhinolaryngol. 2014; 271: 899-904.
- Michel J, Fakhry N, Mancini J, Braustein D, Moreddu E, Giovanni A, et al. Sinonasal Squamous Cell Carcinomas: Clinical Outcomes and Predictive Factors. Int J Oral Maxillofac Surg. 2014; 43: 1-6.
- Kano S, Hayashi R, Homma A, Matsuura K, Kato K, Kawabata K, et al. Effect of Local Ectension Sites on Survival in Locally Advanced Maxillary Sinus Cancer. Head Neck. 2014; 36: 1567-1572.
- Sakashita T, Homma A, Hatakeyama H, Kano S, Mizumachi T, Furusawa J, et al. The Incidence of Late Neck Recurrence in No Maxillary Sinus Squamous Cell Carcinomas after Superselective Intra-Arterial Chemoradiotherapy without Prophylactic Neck Irradiation. Eur Arch Otorhinolaryngol. 2014; 271: 2767-2770.
- Saedi B, Aghili M, Motiee M, Valadkhani S, Niazi AB, Safavi A. Surgical Outcomes of Malignant Sinonasal Tumors: Open versus Endoscopic Surgical Approaches. J Laryngol Otol. 2014; 128: 784-790.