Case Report
Elevated Prothrombin Time/International Normalized Ratio after Concomitant Administration of Erlotinib in Patients Receiving Warfarin: A Report on Two Cases
Hiroyuki Watanabe1*, Yu Nishibatake1, Kojiro Hata1, Yoko Makihara1, Yoichi Nakanishi2 and Satohiro Masuda1
1Department of Pharmacy, Kyushu University Hospital, Japan
2Research Institute for Diseases of the Chest, Kyushu University, Japan
*Corresponding author: Hiroyuki Watanabe, Department of Pharmacy, Kyushu University Hospital, 3-1-1 Maidashi, Higashi-ku, Fukuoka, Fukuoka 8128582, Japan
Published: 09 Dec, 2016
Cite this article as: Watanabe H, Nishibatake Y, Hata K,
Makihara Y, Nakanishi Y, Masuda
S. Elevated Prothrombin Time/
International Normalized Ratio after
Concomitant Administration of Erlotinib
in Patients Receiving Warfarin: A
Report on Two Cases. Ann Clin Case
Rep. 2016; 1: 1210.
Abstract
Adverse interactions between warfarin and cytotoxic agents are well documented; however,
interactions between warfarin and erlotinib are not well characterized. Here we report two patients
with non-small cell lung cancer in whom concomitant treatment with erlotinib and warfarin resulted
in elevated prothrombin time-international normalized ratio (PT-INR) values. In the first case, the
PT-INR value increased from 2.24 to 2.73 9 days after initiating erlotinib treatment in a 59-yearold
Japanese man receiving prophylactic warfarin therapy for cardiovascular disease. However,
erlotinib did not adversely affect the hepatic function in this patient. In the second case, the PTINR
value increased from 1.58 to 7.54 7 days after initiating erlotinib treatment in a 61-year-old
Japanese man receiving prophylactic warfarin therapy for cerebral infarction; this was accompanied
by bleeding diathesis (purpura). In both cases, PT-INR was well maintained by warfarin therapy
prior to initiating erlotinib treatment.
Our experience with these cases underlines the importance of the careful monitoring of the PT-INR
value and appropriate adjustment of warfarin dosage in patients receiving concomitant erlotinib
and warfarin therapy.
Introduction
Drug interactions can cause many clinical complications, particularly when drugs are coadministered
with anticancer agents. Erlotinib, a reversible epidermal growth factor receptor
(EGFR) tyrosine kinase inhibitor (TKI) is commonly used to treat advanced non-small cell lung
cancer (NSCLC). Warfarin, an anticoagulant, interacts with various drugs, resulting in altered
effects.
Thromboembolic phenomena, such as deep vein thrombosis and pulmonary embolism,
commonly occur in cancer patients and often necessitate prophylactic anticoagulant therapy with
warfarin [1]. Owing to the interaction of several drugs with warfarin and the large inter-individual
variability in pharmacodynamics, warfarin dosage must be adjusted according to the prothrombin
time-international normalized ratio (PT-INR) in individual patients.
The prescription information for erlotinib treatment includes a precaution for patients on
concomitant warfarin therapy owing to the associated risk of elevated PT-INR values. However, the
underlying mechanism of this interaction is unknown. Although a few reports have described the
interaction between gefitinib and warfarin [2], the interaction between warfarin and erlotinib is not well characterized. Here we report two patients with lung cancer, who exhibited an increase in the
PT-INR values after concomitant administration of erlotinib and warfarin.
Case Presentation
Case 1
A 59-year-old Japanese man was diagnosed as having lung non-squamous cell adenocarcinoma
(stage IV: T1N3M1). He was maintained on a regular warfarin therapy (3.5 mg daily) for prophylaxis
from cardiac infarction. After three courses of chemotherapy with carboplatin and pemetrexed, he
exhibited a grade 3 reduction in hemoglobin and a decline in renal function. Therefore, secondline
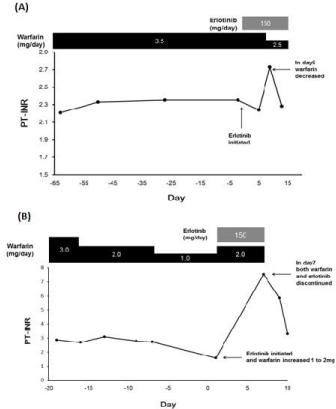
chemotherapy was initiated with erlotinib at the dose of 150 mg daily (day 1). His PT-INR value was relatively stable (2.1-2.4) and the daily warfarin dosage
was maintained at 3.5 mg for at least 3 months before erlotinib was
initiated. Nine days after initiating erlotinib therapy, the patient’s PTINR
value increased from 2.24 to 2.73. Therefore, starting from day
9, the warfarin dose was decreased to 2.5 mg daily. On day 13, his
PT-INR value restored to 2.24, and he continued receiving warfarin
at the dose of 2.5 mg (Figure 1A). Aspartate aminotransferase (AST),
alanine aminotransferase (ALT), serum creatinine (SCr), and serum
albumin (Alb) levels did not change after treatment with erlotinib.
There were no changes in diet or severe episodes of nausea or
vomiting.
Case 2
A 61-year-old Japanese man was diagnosed as having lung
non-squamous cell adenocarcinoma (stage IV: T4N3M1). He was
on warfarin therapy for multiple cerebral infarcts. Warfarin dosage
was adjusted to maintain a target PT-INR value of 2.0-2.5. His PTINR
value was relatively stable (1.8-2.5) when 3 mg warfarin was
administered daily for at least 1 month before initiating the sixthline
chemotherapy comprising a daily dose of 150-mg erlotinib (day
1). On day 19, before initiating erlotinib therapy, his PT-INR value
was 2.86; thus, warfarin dose was reduced to 2 mg. On day 9, before
initiating erlotinib therapy, the PT-INR value was still high (2.79),
which warranted a further dose reduction to 1 mg. On day 1, after
initiating erlotinib, the PT-INR value decreased to 1.58; thus, from
day 2, warfarin dosage was increased to 2 mg daily.
However, 7 days after initiating erlotinib therapy, the PT-INR
value increased to 7.54 (Figure 1B). The patient developed purpura on the arms. Warfarin was discontinued, and the anticoagulant effect
was rapidly reversed by administering subcutaneous phytonadione.
Owing to the decline in the renal and hepatic functions (day 1: SCr,
1.09 mg/dL; AST, 49 U/mL; ALT, 29 U/mL → day 7: SCr, 1.52 mg/dL;
AST, 99 U/mL; ALT, 46 U/mL), erlotinib treatment was discontinued.
Three days later (day 10), the PT-INR value decreased to 3.3. Both
patients took the prescribed warfarin dose every day without fail.
Figure 1
Figure 1
Changes in prothrombin time/international normalized ratio (PTINR)
after concomitant administration of erlotinib and warfarin (A) Clinical
case 1. The patient had been receiving warfarin at a dose of 3.5 mg/day
for 4 months before initiating erlotinib treatment. (B) Clinical case 2. The
patient was administered warfarin at a dose of 3 mg/day for 6 months before
initiating erlotinib treatment.
Discussion
Owing to the relatively advanced age at the diagnosis of lung
cancer, most patients are likely to receive anticoagulant therapy for
pre-existing cardiovascular diseases [1,2]. Prophylactic or therapeutic
warfarin therapy is often recommended for cancer patients. Warfarin
has a narrow therapeutic index, which makes it the third most
common drug responsible for hospital admissions because of adverse
drug effects [3]. The interaction of several drugs with warfarin elevates
the risk of thrombotic or hemorrhagic events in such patients [4-5].
We report two cases wherein the co-administration of erlotinib and
warfarin potentiated the effect of warfarin.
In case 1, the PT-INR value increased 9 days after initiating
erlotinib treatment. Kelly S et al. [6] demonstrated an increase in the
PT-INR value 7 days after initiating erlotinib, which necessitated a
decrease in the warfarin dose. As a result, in this case, the first blood
examination was performed on day 5, when the PT-INR value was
relatively stable. However, blood examination at an earlier stage is
required to establish the time-point at which the PT-INR value begins
to rise after initiating erlotinib along with warfarin therapy. In case
2, warfarin dosage was increased at the time of initiating erlotinib,
which resulted in a sudden increase in the PT-INR value. If a sudden
decline in the renal or hepatic functions causes a sudden rise in the
PT-INR value, as in this case, it is necessary to consider an increase in
the warfarin concentration in the blood.
The exact mechanism underlying this interaction is yet unknown;
however, it is possibly related to a decrease in warfarin metabolism
via the inhibition of the cytochrome P450 (CYP) enzyme system by
erlotinib or one of its metabolites. Warfarin is present as a racemic
mixture, with each enantiomer differentially metabolized by the
liver microsomal system. The R-enantiomer is metabolized by
CYP1A2/3A4, whereas the S-enantiomer is primarily metabolized
by CYP2C9. The efficacy of warfarin is mainly affected by the altered
metabolism of the S-enantiomer; however, the R-form has a longer
half-life and is a noncompetitive inhibitor of S-warfarin metabolism
by CYP2C9 [7-8]. Erlotinib is metabolized in the liver, mainly by
cytochrome CYP 3A4/3A5, and to a lesser extent, by CYP1A1/1A2.
Because the metabolizing enzyme of erlotinib resembles that of the
R-form of warfarin, it is likely that the competitive inhibition of
warfarin metabolism is responsible for the increase in the warfarin
concentration in the blood. It is interesting that afatinib was hardly
metabolized by CYP, unlike other EGFR-TKIs. Future data on the
concomitant administration of afatinib and warfarin may help
elucidate the mechanism of interaction between warfarin and EGFR-TKIs.
According to the package inserts of warfarin and erlotinib, both
drugs are highly plasma protein bound (warfarin: 97%, erlotinib:
95%). According to the product information of erlotinib, an in vitro
study that examined the impact of erlotinib on the plasma protein
binding of warfarin showed a little effect on the binding capacity of
warfarin; the underlying mechanism of this interaction is unclear. It is likely that erlotinib may also compete with warfarin for the albuminbinding
sites, which may result in elevated levels of unbound warfarin,
and a consequent potentiation of hypoprothrombinemia. Therefore,
pharmacokinetic and pharmacodynamic interactions seem to occur
between erlotinib and warfarin. Liver disease, kidney disease, and
hypoalbuminemia are known risk factors for bleeding in patients
on warfarin therapy [9,10]. In the phase I pharmacokinetic studies
of erlotinib, patients with renal dysfunction could tolerate 150 mg of
erlotinib daily and were found to have erlotinib clearance similar to
that in patients with normal renal function. However, patients with
hepatic dysfunction should receive a lower dose consistent with their
reduced clearance [11]. In case 1, the co-administration of warfarin
and erlotinib did not affect the renal and hepatic functions; however,
in case 2, a decline in the renal and hepatic functions occurred after
initiating erlotinib. Therefore, this probably shows the potential
influence of erlotinib on the decline in the hepatic functions. Camidge
et al. [12] demonstrated an increase in the area under the curve of
warfarin when warfarin and capecitabine were co-administered. We
did not measure the warfarin concentrations in the blood in the two
cases. It is likely that erlotinib increased the warfarin concentrations
in the blood via the inhibition of its metabolism by CYP 3A4,
which potentiated its anti-coagulant effects. Warfarin therapy has a
detrimental effect on cancer patients. During warfarin maintenance
therapy, approximately one-third of the patients experience side
effects, such as recurrence and bleeding [13]. In a recent study,
erlotinib alone or in combination with bevacizumab, as the firstline
therapy, was shown to have a favorable effect in patients with
advanced NSCLC [14]. Bevacizumab therapy is associated with a risk
of bleeding. Therefore, the screening of patients on warfarin therapy
is of key importance prior to initiating erlotinib. The concomitant
administration of erlotinib and warfarin requires patient education
regarding the risk of bleeding and the need for a close monitoring of
PT-INR for necessary warfarin dose adjustment.
Conclusion
The concomitant administration of erlotinib and warfarin resulted in an increase in the PT-INR values, which suggests the need for the careful monitoring of the PT-INR values and appropriate adjustment of warfarin dosage.
Acknowledgment
This work was supported by a JSPS KAKENHI Grant Number JP16K08404.
References
- Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013; 122: 1712-1723.
- Arai S, Mitsufuji H, Nishii Y, Onoda S, Ryuge S, Wada M, et al. Effect of gefitinib on warfarin antithrombotic activity. Int J Clin Oncol. 2009; 14: 332-336.
- Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011; 365: 2002-2012.
- Le AT, Hasson NK, Lum BL. Enhancement of warfarin response in a patient receiving etoposide and carboplatin chemotherapy. Ann Pharmacother. 1997; 31: 1006-1008.
- Saif MW. An adverse interaction between warfarin and fluoropyrimidines revisited. Clin Colorectal Cancer. 2005; 5: 175-180.
- Thomas KS, Billingsley A, Amarshi N, Nair BA. Elevated international normalized ratio associated with concomitant warfarin and erlotinib. Am J Health Syst Pharm. 2010; 67: 1426-1429.
- Kaminsky LS, Zhang ZY. Human P450 metabolism of warfarin. Pharmacol Thera. 1997; 73: 67-74.
- Porter WR. Warfarin: history, tautomerism and activity. J Comput Aided Mol Des. 2010; 24: 553-573.
- DiMarco JP, Flaker G, Waldo AL, Corley SD, Greene HL, Safford RE, et al. Factors affecting bleeding risk during anticoagulant therapy in patients with atrial fibrillation: observations from the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Am Heart J. 2005; 149: 650-656.
- Abdelhafiz AH, Myint MP, Tayek JA, Wheeldon NM. Anemia, hypoalbuminemia, and renal impairment as predictors of bleeding complications in patients receiving anticoagulation therapy for nonvalvular atrial fibrillation: a secondary analysis. Clin Ther. 2009; 31: 1534-1539.
- Miller AA, Murry DJ, Owzar K, Hollis DR, Lewis LD, Kindler HL, et al. Phase I and pharmacokinetic study of erlotinib for solid tumors in patients with hepatic or renal dysfunction: CALGB 60101. J Clin Oncol. 2007; 25: 3055-3060.
- Camidge R, Reigner B, Cassidy J, Grange S, Abt M, Weidekamm E, et al. Significant effect of capecitabine on the pharmacokinetics and pharmacodynamics of warfarin in patients with cancer. J Clin Oncol. 2005; 23: 4719-4725.
- Prandoni P, Lensin AW, Piccioli A, Bernardi E, Simiori P, Girolami B, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002; 100: 3484-3488.
- Seto T, Kato T, Nishio M, Goto K, Atagi S, Hosomi Y, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol. 2014; 15: 1236-1244.