Case Report
An Unusual Manifestation of Pancreatic Cancer
Jūratė Gudaitytė* and Agnė Kaunienė
Department of Anaesthesiology, Lithuanian University of Health Sciences, Lithuania
*Corresponding author: Jūratė Gudaitytė, Department of Anaesthesiology, Medical Academy, Lithuanian University of Health Sciences, LT 50009 Kaunas, Lithuania
Published: 08 Dec, 2016
Cite this article as: Gudaitytė J, Kaunienė A. An Unusual
Manifestation of Pancreatic Cancer.
Ann Clin Case Rep. 2016; 1: 1207.
Abstract
The following case presents an atypical manifestation of pancreatic cancer, the latter being diagnosed only after biopsy results. The patient complaints and initial tests led to the preliminary diagnosis of acute pancreatitis which occurred after a dietary change. Moreover, after arrival to the hospital this patient had E.coli in his blood sample. Inflammatory parameters were not increased. However, he didn’t have any symptoms of sepsis. The blood sample was repeated, and again the result showed positive E.coli. The antibacterial and other symptomatic treatment seemed to be effective for the first 4 days. However, on the 29th day after the first signs of the disease, the patient became unresponsive and died due to respiratory and cardiovascular failure. The final diagnosis appeared to be a pancreatic stage 1 cancer in the head. The patient died because of sepsis, multiple organ failure, and cardiopulmonary insufficiency.
Keywords: Acute pancreatitis; Pancreatic cancer; E.coli
Case Presentation
The subject of this report was a 78-year-old white, married, non-drinking, non-smoking male,
physically active (he used to travel about 30 km by bicycle every day) and on a healthy diet. However,
his eating habits changed when he was in Italy. When he came back to Lithuania, he felt sick. He felt
pain in the upper abdominal zone and indigestion, had icterus, his urine was brown.
Anamnesis of this patient: the patient had a grade IV mitral valve leakage, no allergies and no
surgeries. The blood test and an abdominal ultrasound were done on the first day in the hospital.
Treatment and diagnostics
Results of the first day (June 21, 2016) blood samples were as follows: blood count WBC 7.99
μU/ml, RBCs 3.81 μU/ml, Hgb 114 g/l ↓, PLT 222 μU/ml; blood chemistry ALP 911U/l↑, ALT
372 U/l↑, AST 280 U/l↑, total bilirubin 168.9 mmol/l↑, urea 7.6 mmol/l, creatinine 96.4 μmol/.l,
P-amylaze 187U/l↑, CRP 3.6 mg/l; blood culture positive E.coli.
The following data was found during abdominal ultrasound: the liver surface smooth,
homogenous, liver of normal size. Intrahepatic gall-bladder ducts were dilated in both lobes; ductus
choledochus was normal and seemed to be clear. The gall-bladder was large (125 x 49 mm), clear and
with thin walls. Pancreas could not be seen because of flatulence. The findings led to the diagnosis of
intra- and extrahepatic cholestasis and cholecystitis.
After blood tests and abdominal ultrasound the doctors decided to do the ERCP (endoscopic
retrograde cholangiopancreatography). General anaesthesia has been done and the patient has been
considered as having ASA II. Anaesthesia was uneventful. The surgeon used the ERCP protocol and
introduced a stent in the ductus choledochus, because he saw black coloured bile during the dilation
of the ductus.
The patient was treated with antibiotics: cefuroxime 1.5 g x 3 times/d and metronidazole 0, 5 g
x 3 times/d, because of the E.coli bacteria found in the blood sample. Besides, infusion therapy has
been applied and analgesics have been given to the patient.
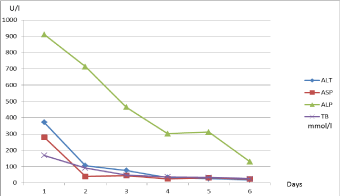
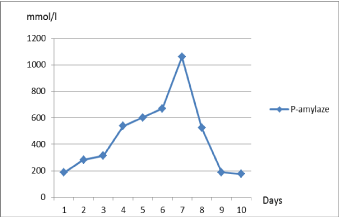
The next two days the levels of liver enzymes decreased (Figure 1), but P-amylase increased from to 187→283 mmol/l (Figure 2). The patient felt better, jaundice disappeared.
After four days (June 21, 2016) the patient complained about feeling sick. CRP increased from
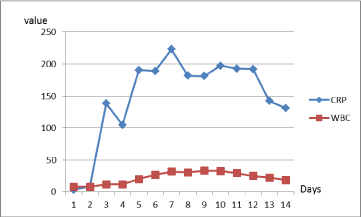
3.6 to 138.8 mg/l, P-amylase from 283 to 314 U/l, urea from 7.6 to 13.4 mmol/l. Other blood
tests were normal and liver enzymes decreased further. The decision was made to proceed with
monitoring the patient's condition and further assessment of the tests.
Four more days after (June 29, 2016), the patient felt pain in the abdominal cavity and complained about feeling weak. CRP increased from 138.8 to 190.2 mg/l (Figure 3), P-amylase from 314 to 539 U/l, WBC from 7.99 to 19.99 μU/ml (Figure 3); other blood and chemistry tests were normal. The surgeon
decided to perform abdominal CT. CT findings were as follows:
hepatic haemangioma in S6, acute pancreatitis, hydrothorax in both
pleural cavities and pleuropneumonia. An additional antibiotic
gentamicin 240mg daily was given i/v. The patient was stable, but felt
sick and weak.
Five days after (July 4, 2016) the patient had hemodynamic
instability, arterial hypotension, tachycardia, paroxysms of atrial
fibrillation, dyspnea and needed supplementary oxygen via nasal
cannula. The patient’s stomach was strained, painful, no peristalsis
could be heard. The surgeon did the abdominal ultrasound and
found fluid near the liver diaphragmatic surface. An ultrasound
guided puncture of the intra-abdominal cavity was made under local
anaesthesia and purulent fluid was extracted. The fluid culture test
revealed the presence of E.coli.
Laparotomy was made because of suspected peritonitis. During
surgery, fluids were found near the gall-bladder, pancreas, in the
Douglas cavity. The pancreas revision has been accomplished;
necrosis has been noticed near the head.
The patient’s haemodynamic and respiratory state were unstable:
he had paroxysmal AF requiring vasopressors and mechanical
ventilation of the lungs. Blood tests revealed the state of sepsis: WBC 31.62 μU/ml, procalcitonin 2.4μg/l. Blood culture has been found: E.coli.
The patient’s condition was poor. His vital signs were stable, but
the blood tests demonstrated a worsening condition. Even more, he
developed acute kidney failure (creatinine 147.2 μmol/l, urea 27.2
mmol/l), the respiratory system and general state were declining.
It was decided to transfer the patient from the secondary to
the tertiary health care centre, Hospital of Lithuanian University of
Health Sciences for treatment and extensive research. During the
transportation the patient was stable and could breathe by himself.
There was no fever, but the patient felt pain in the upper abdominal
area with no signs of peritoneum irritation. The patient was
transferred to the Department of Surgery (July 13, 2016).
After five days (July 18, 2016) CT scan was repeated: the size
of the liver was normal, but multiple hypodense focuses resembled
abscesses. Choledochal stent was in the correct position, the gallbladder
was normal. Pancreatic tissue had no normal structure,
ductus pancreaticus was dilated approximately to 1 cm, and mini
calcinations could be seen. Traces of fluid were visible near the
pancreas. After CT evaluation it was decided to change treatment:
vancomycin 1 g x 1/d (adjusted for GFR) and meropenem 1gx3/d
were started.
After two days (July 20, 2016) inflammatory indicators elevated
and the patient’s condition did not improve. It was decided to repeat
the ERCP. During the procedure, debris mass was removed as well
as the stent. It was decided to take a biopsy of Papilla Wateri and an
extra bile sample for microbial growth. The answer of the emergency
biopsy was adenocarcinoma of pancreas.
The patient’s condition was severe, but stable. He complained
having dyspnea. The doctor was able to hear wheezes. Then the
bronchoscopy has been done. A pus-looking fluid has been detected
in both of the bronchi. This confirmed bronchial aspiration and
samples from bronchi microbial growth were taken.
Finally (July 22, 2016) the microbiological tests of the bile
revealed growth of E.coli (responsive to karbapenem), acinetobacter
baumannii (responsive to kolistin), E faecium (responsive particularly
linezolid), Enterococcus faecalis (responsive to vancomycin).
Inflammatory indicators declined, so it was decided to continue the
same treatment. Unexpectedly on the same day, the patient developed apnea, and resuscitation according to asystole algorithm has been applied immediately. After successful resuscitation the patient was
transferred to the ICU, but he died two hours later.
Clinical diagnosis
Pancreatic cancer (stage I), Acute pancreatitis, Mechanical icterus,
Acute cholangitis, Multiple liver abscesses, Pleuropneumonia, Sepsis,
Septic shock (E.coli), Cardiopulmonary insufficiency.
Figure 1
Figure 1
Dynamics of liver enzymes.
ALT (Alanine transaminase, U/l, normal range 0-55), ASP (Aspartate
transaminase, U/l normal range 5-34), ALP (Alkaline phosphatase, U/l,
normal range 40-150), TB (Total bilirubin, mmol/l, normal range 3,4-20,5).
Figure 2
Figure 3
Figure 3
Dynamics of inflammatory indicators.
CRP (C-reactive protein, mg/l, normal range 0-5), WBC (white blood cells,
μU/ml, normal range 4-10).
Discussion
This clinical case is interesting, because the disease did not
have any typical signs of pancreatitis: there were no winding pain,
nausea or vomiting, inflammation indicators have been within the
normal range. In the literature, acute pancreatitis is described having
the following symptoms: fever (76%) and tachycardia (65%) [1-5].
The patient did not have these symptoms. He had indigestion, pain
and jaundice. It is also interesting that the patient became sick after
a dietary change. Rare reasons for the pancreatitis described in the
literature might be high levels of fat and calcium in the blood [6].
Currently we have found no publications related to the changes of the
diet that could lead to pancreatitis. Several publications describe high
levels of triglycerides, hypercalcemia. Mortality in acute pancreatitis
is usually due to systemic inflammatory response syndrome and
organ failure in the first two-week period, while after two weeks it
is usually due to sepsis and its complications [7,8]. Provided that
the patient had not developed acute pancreatitis and pancreatitisassociated
complications, perhaps he would not have been diagnosed
stage 1 pancreatic cancer. Approximately 75 % of all pancreatic
carcinomas occur within the head or neck of the pancreas, 15-20 %
occurs in the body of the pancreas, and 5-10 % occurs in the tail [9].
In most cases of pancreatic cancer, acute pancreatitis manifests where
cancer is widespread and causes obstruction. However, data that
acute pancreatitis is a risk factor for pancreatic cancer is very limited.
The risk of acute pancreatitis in the case underlying pancreatic cancer
was estimated to be approximately of 1.5% [10,11]. Pancreatic cancer
is the fourth leading cause of cancer deaths, being responsible for 7
% of all cancer-related deaths for men and women. Pancreatic cancer
does not have any early symptoms; the symptoms appear when the
cancer spreads [12]. Finally, this patient was a good lesson that not
necessarily an advanced cancer cause acute pancreatitis, but even a
small stage cancer can complicate everything. Cancer depresses the
immune system. Cancer cells may: reduce the expression of tumor
antigens on their surface, making it harder for the immune system
to detect them; express proteins on their surface that induce immune
cell inactivation; induce cells in the surrounding environment
(microenvironment) to release substances that suppress immune
responses and promote tumor cell proliferation and survival [13]. The
inactivation of the immune system could be the cause of death of this
particular patient.
If the patient had been treated in the Intensive Care Unit instead
of keeping him in the Department of Surgery he might have had
a different outcome now. Initially the patient state was severe, but
stable. When the number of patients who require intensive care is
greater than the number of beds available, Intensive Care Unit (ICU)
entry flow is obstructed [14]. The waiting time for ICU bed availability
varies between hospitals and countries, and typically ranges from 2
hours to 3.5 days [15-20]. Finally the disease progressed to sepsis and
septic shock. The patient died due to cardiorespiratory failure 29 days
after initial complaints. The patient was treated for 29 days after initial
symptoms and the patient care seemed to be adequate for his health care providers in the secondary and tertiary levels.
However, the sudden determination of his state was not noticed in
the surgical ward having limited monitoring possibilities. It remains
unclear why the patient was not transferred to the ICU earlier. Most
studies of ICU triage have focused on patients admitted or rejected
for ICU management which prevents comparison with patients of
late transfers to the ICU [21]. Late admission to the ICU is associated
with increased rates of mortality.
In conclusion, this was a rare case of an early, stage 1 pancreatic
cancer manifesting as an acute pancreatitis and complicated by sepsis,
multiple organ failure and death. There was no suspicion of cancer
initially; this was diagnosed only after a biopsy. Having in mind
the early stage of cancer, we presume it would have been curable.
However, it was the sepsis induced by acute pancreatitis and sepsisinduced
multiple organ failure that led to unfavourable outcomes.
By bringing attention to the unexpected lethal outcome of this case
we want to highlight the need of more extensive monitoring of the
patient’s state in cases of acute pancreatitis and septic complications.
References
- Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis 2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013; 62: 102-111.
- Granger J, Remick D. Acute pancreatitis: models, markers, and mediators. Shock. 2005; 24: 45-51.
- Banks PA. Epidemiology, natural history, and predictors of disease outcome in acute and chronic pancreatitis. Gastrointest Endosc. 2002; 56: S226-230.
- Whitcomb DC. Clinical practice. Acute pancreatitis. N Engl J Med. 2006; 354: 2142-2150.
- Frick TW, Fryd DS, Sutherland DE, Goodale RL, Simmons RL, Najarian JS. Hypercalcemia associated with pancreatitis and hyperamylasemia in renal transplant recipients. Data from the Minnesota randomized trial of cyclosporine versus antilymphoblast azathioprine. Am J Surg. 1987; 154: 487-489.
- Gloor B, Müller CA, Worni M, Martignoni ME, Uhl W, Büchler MW. Late mortality in patients with severe acute pancreatitis. Br J Surg. 2001; 88: 975-979.
- Mutinga M, Rosenbluth A, Tenner SM, Odze RR, Sica GT, Banks PA. Does mortality occur early or late in acute pancreatitis?. Int J Pancreatol. 2000; 28: 91-95.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Pancreatic Adenocarcinoma, v.2.2015.
- Munigala S, Kanwal F, Xian H, Scherrer JF, Agarwal B. Increased risk of pancreatic adenocarcinoma after acute pancreatitis. Clin Gastroenterol Hepatol. 2014; 12: 1143-1150.e1
- Schreiber RD, Old LJ, Smyth MJ. Cancer Immunoediting: Integrating Immunity’s Roles in Cancer Suppression and Promotion. Science. 2011: 331: 1565-1570.
- Levin PD, Sprung CL. The process of intensive care triage. Intensive Care Med. 2001; 27: 1441-1445.
- What you need to know about cancer of the pancreas. National Cancer Institute.
- Chiavone PA, Rasslan S. Influence of time elapsed from end of emergency surgery until admission to intensive care unit, on Acute Physiology and Chronic Health Evaluation II (APACHE II) prediction and patient mortality rate. Sao Paulo Med J. 2005; 123: 167-174.
- Chalfin DB, Trzeciak S, Likourezos A, Baumann BM, Dellinger RP, DELAY-ED Study Group. Impact of delayed transfer of critically ill patients from the emergency department to the intensive care unit. Crit Care Med. 2007; 35: 1477-1483.
- Young MP, Gooder VJ, McBride K, James B, Fisher ES. Inpatient transfers to the intensive care unit: Delays are associated with increased mortality and morbidity. J Gen Intern Med. 2003; 18: 77-83.
- Sprung CL, Geber D, Eidelman LA, Baras M, Pizov R, Nimrod A, et al. Evaluation of triage decisions for intensive care admission. Crit Care Med. 1999; 27: 1073-1079.
- Giordano A, Moraes L, Iturralde A, Cancela M. Demanda de camas en medicina intensiva. Proceso de ingreso al centro de tratamientos intensivos del Hospital de Clínicas durante un mês. Rev Med Urug. 2007; 23: 40-49.
- American Hospital Association Annual Data Exchange Survey: October 6, 2016.
- Duke G, Green J, Briedis J. Survival of critically ill medical patients is timecritical. Crit Care Resusc. 2004; 6: 261-267.
- Parkhe M, Myles PS, Leach DS, Maclean AV. Outcome of emergency department patients with delayed admission to an intensive care unit. Emerg Med (Fremantle). 2002; 14: 50-57.
- Cardoso LTQ, Grion CMC, Matsuo T, Anami EHT, AM Kauss IAM, Seko L, et al. Impact of delayed admission to intensive care units on mortality of critically ill patients: a cohort study. Critical Care 2011; 15: R28.