Case Report
Endometrial Adenosarcoma: Case Report
Elizabeth Parker1, Julie Middleton1, Rochelle L Garcia2, Mark R Kilgore2 and Renata R Urban3*
1Department of Psychiatry and Behavioral Sciences, University of Washington School of Medicine, USA
2Department of Pathology, University of Washington Medical Center, USA
3Department of Obstetrics and Gynecology, University of Washington Medical Center, USA
*Corresponding author: Renata R Urban, Department of Obstetrics & Gynecology, University of Washington Medical Center, 1959 NE Pacific Street, Campus Box 356460, Seattle, WA 98195-6460, USA
Published: 01 Nov, 2016
Cite this article as: Parker E, Middleton J, Garcia RL,
Kilgore MR, Urban RR. Unusual
Presentation of a Metastatic
Choriocarcinoma: A Case Report of
Non-Gestational Choriocarcinoma
Presenting as Advanced Colorectal
Cancer. Ann Clin Case Rep. 2016; 1:
1173.
Abstract
Case: A 51-year-old perimenopausal female presented with persistent vaginal bleeding, elevated
human chorionic gonadotropin (hCG), and colonic and liver masses. Her initial diagnosis was felt
to be metastatic colorectal cancer. Given that her hCG was rising, her pathology was re-reviewed
and felt to be consistent with choriocarcinoma. To date, she received three cycles of EMA-CO
chemotherapy with resultant drop in hCG.
Conclusion: This patient’s presentation and subsequent diagnosis with metastatic choriocarcinoma
highlight the importance of evaluating a gynecologic complaint noted in the context of a diagnosis of
advanced cancer. Although the initial presentation with anemia in the setting of colorectal and liver
masses painted a clinical picture raising concern for metastatic colorectal cancer, the significantly
elevated hCG level found in the investigation of irregular bleeding requires additional evaluation.
This case report emphasizes the importance of considering a diagnosis of choriocarcinoma even in
patients with remote or unclear history of pregnancy.
Keywords: Gestational trophoblastic disease; Hcg; EMA-CO; Choriocarcinoma; Colon cancer
Introduction
Colorectal cancer is the third most common cancer in the United States with an incidence
of nearly 136,000 cases each year. A common presentation is iron deficiency anemia secondary
to blood loss from cancerous lesions. Nearly 95% of colorectal cancer is adenocarcinoma with
varying morphologic features and cell differentiation. A number of risk factors contribute to
the accumulation of mutations that are present in colorectal neoplasia. Obesity, western diet,
inflammatory bowel disease, family history, and genetic predisposition are all thought to factor into
the stepwise progression of mutations transforming adenomas to adenocarcinoma. In the advanced
stage of disease, metastasis is most common to the liver through hematogenous spread from venous
blood supply [1].
In contrast to this common malignancy, the incidence of choriocarcinoma is 1 in 20-40,000
pregnancies. The diagnosis is made by rising hCG in the absence of a gestation and histology, if
available. Because the tumors are highly vascular, biopsy of metastases is not recommended due to
bleeding risk. Presenting symptoms include vaginal bleeding, dysmenorrhea, and focal symptoms
related to common areas of metastasis such as hemoptysis, vaginal bleeding, and focal neurologic
symptoms. Metastatic spread is common for this highly vascular malignancy, and at presentation
80% of patients have lung involvement, 30% vaginal involvement, 10% liver involvement, and 10%
central nervous system involvement. Not all choriocarcinomas are preceded by a gestational event,
and can be gestational (half molar pregnancies and half non-molar pregnancies) or non-gestational
[2]. Primary ovarian choriocarcinoma is exceedingly rare [3].
The management of high-risk metastatic gestational trophoblastic neoplasms such as
choriocarcinoma (stage II/III with prognostic score ≥7 and stage IV) includes multi-agent
chemotherapy with possible radiation or surgery depending on involved sites and is associated
with 80-90% survival [4]. Early management included MAC, methotrexate, actinomycin-D, and
cyclophosphamide with a 63-71% cure rate. In the 1980s, management evolved into CHAMOCA (cyclophosphamide, hydroxyurea, actinomycin D, methotrexate with
folic acid, vincristine, doxorubicin) although this combination was
problematic with significant toxicity. Management further evolved
with the recognition of the activity in treating GTN with etoposide.
Subsequent therapy with EMA-CO (etoposide, methotrexate with
folic acid, actinomycin D, cyclophosphamide, vincristine) resulted in
improved survival rates [5].
Case Presentation
A 51-year-old G1P0010 perimenopausal woman was in her usual
state of good health until experiencing increasingly heavy menses in
2015. She presented as an outpatient to her primary care physician,
was found to be anemic, and was treated with iron. In January 2016,
she again experienced persistent vaginal bleeding. She presented again
in April to care and was prescribed Provera. She was then referred to
an obstetrician/gynecologist.
She felt well until June 2016 when she developed right upper
quadrant pain and presented to a local emergency room. Her
initial evaluation included an elevated serum human chorionic
gonadotropin (hCG) of 18,000, but a pelvic ultrasound revealed an
empty uterus and unremarkable adnexa. A subsequent CT of the
abdomen and pelvis revealed a mass in the ascending colon, enlarged
mesenteric lymph nodes and multiple hypodense liver lesions. A PET/
CT scan confirmed a hypermetabolic mass in the ascending colon and
mesentery, multiple liver masses and mesenteric and retroperitoneal
lymphadenopathy. There were also hypermetabolic peritoneal and
pelvic deposits including in the adnexa. Colonoscopy revealed a
partially obstructive ascending colonic mass which was biopsied.
The pathology was read as poorly differentiated adenocarcinoma of
the ascending colonic mass and tubulovillous adenoma of the polyp.
The patient was diagnosed with presumed colorectal carcinoma
and plans were made to start a FOLFOX chemotherapy regimen.
During this time, her hCG was rechecked and was found to have risen to 96,000. Given the discrepancy between the rising hCG and
tumor presentation, the pathology was reviewed at a tertiary care
center and was felt to be consistent with choriocarcinoma (Figure 1).
Immediately after diagnosis, the patient was brought in for emergent
initiation of chemotherapy with EMA-CO. Additional evaluation
prior to chemotherapy included a brain MRI which showed no
evidence of cerebral disease.
Upon further discussion, her gynecologic history included
menarche at age 12 years with irregular and painful menses, and an
elective abortion at age 16 without additional pregnancies. She used
oral contraception between the age of 16 to 40, and used condoms for
birth control.
The morphologic and immunohistochemical findings in
this clinical scenario are most consistent with a diagnosis of
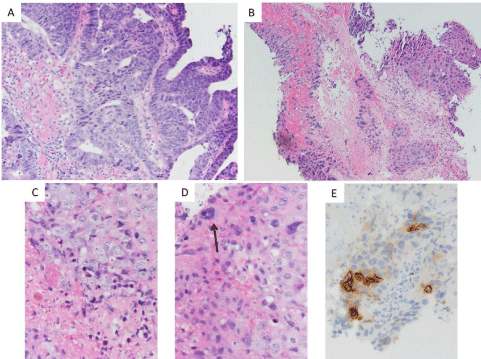
choriocarcinoma. All photos are taken with a 0.55 mm objective
and an Olympus DP73 camera, A-D is photos of H&E-stained slides
taken at the indicated power.
Figure 1
Figure 1
(A) Representative foci more reminiscent of colorectal-type neoplasm (10x). (B) Representative foci of extensively necrotic high-grade neoplasm with
associated necrotic debris (10x). (C) Foci of probable cytotrophoblast-like neoplastic cells with associated apoptotic bodies and necrotic debris (40x). (D) Focus
of neoplasm with multinucleated cell (arrow) suggestive of syncytiotrophoblast-like neoplastic cells intermixed with probable cytotrophoblast-like neoplastic cells,
hemorrhage, and apoptotic bodies (40x). (E) Beta-HCG immunohistochemical stained slide revealing immunoreactivity in a subset of neoplastic cells.
Discussion
This patient’s presentation and subsequent diagnosis with
metastatic choriocarcinoma highlights certain clinical concepts. Even
in a perimenopausal female, incorporating hCG into the evaluation
of abnormal vaginal bleeding is appropriate. In addition, an elevated
hCG in the absence of pregnancy should raise serious concerns for
a gestational trophoblastic neoplasm or ovarian germ cell tumor.
Although the patient’s initial presentation with anemia in conjunction
with colon mass and hepatic metastases raised concern for metastatic
colorectal cancer, a rising hCG level cannot be ignored.
This case report emphasizes the importance of considering a
diagnosis of choriocarcinoma even in patients with remote or unclear
history of pregnancy. The differentiation of gestational versus nongestational
choriocarcinoma is useful in guiding management and providing prognostic information to patients. Because gestational
choriocarcinomas arise from gestational tissue, the tumors boast
paternal DNA, with or without maternal DNA. Nongestational
choriocarcinoma is a malignant transformation of trophoblastic
cells unrelated to a gestational event. As such, the tumors boast
maternal DNA without paternal DNA. Importantly, nongestational
choriocarcinoma is highly aggressive, more so than gestational
choriocarcinoma, and all cases of nongestational choriocarcinoma
are treated with multiagent chemotherapy regardless of stage and
prognostic score [6].
Genetic technology has advanced such that reliable assays using
PCR-based DNA analysis of microsatellite markers or short tandem
repeat analysis of genetic material can correctly identify if tumor
DNA is paternal, maternal or both [7,8]. A good clinical history
may mitigate the need to distinguish between gestational and nongestational
choriocarcinoma, however that is not always the case.
In 2014 Buza et al. [6] reported a case of a 22 year old G1P1 who
presented with her last menstrual period 10 weeks prior, elevated
hCG to >200,000 and a right adnexal mass. Upon DNA genotyping
she was determined to have nongestational choriocarcinoma. The
patient did well after surgical resection and multiagent chemotherapy
with EMA-EP (etoposide, metotrexate, actinomycin D, and cisplatin
[6].
Regarding management, nongestational choriocarcinoma is
thought to be more aggressive, warranting multiagent chemotherapy
regardless of stage [2]. Distinguishing gestational versus
nongestational choriocarcinoma would be important if the patient
has stage I or stage II/III disease with a low prognostic score disease,
since these patients would receive single agent chemotherapy per
current guidelines. However, choriocarcinoma is an aggressive tumor
often presenting with metastases, so many patients receive multiagent
chemotherapy and management does not change by distinguishing
gestational versus nongestational disease.
Conclusion
For this patient, the measurement of hCG and subsequent followup values in the context of irregular bleeding was critical in providing the unusual diagnosis in what seemed to be an advanced colorectal carcinoma. Without this and careful review of pathology slides, the diagnosis of choriocarcinoma would not have been made. Emergent and immediate initiation of chemotherapy drastically improves the prognosis of advanced choriocarcinoma. Whether the distinction of gestational vs non-gestational choriocarcinoma is important to the management of disease through DNA genotyping is not yet known, although it would be most important in stage I or stage II/II with a low prognostic score so the proper chemotherapy is administered.
Author’s Contribution
EP was involved with this patient’s initial admission and the writing of this manuscript. JM was involved with writing this manuscript. RU provided guidance, mentorship, and editing. RG and MK provided guidance, pathologic expertise and micrographs.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2016; 66: 7-30.
- Lurain JR. Gestational trophoblastic disease I: epidemiology, pathology, clinical presentation and diagnosis of gestational trophoblastic disease, and management of hydatidiform mole. Am J Obstet Gynecol. 2010; 203: 531-539.
- Vance RP, Geisinger KR. Pure nongestational choriocarcinoma of the ovary. Report of a case. Cancer. 1985; 56: 2321-2325.
- Lurain JR. Gestational trophoblastic disease II: classification and management of gestational trophoblastic neoplasia. Am J Obstet Gynecol. 2011; 204: 11-18.
- Newlands ES, Bagshawe KD, Begent RH, Rustin GJ, Holden L. Results with the EMA/CO (etoposide, methotrexate, actinomycin D, cyclophosphamide, vincristine) regimen in high risk gestational trophoblastic tumours, 1979 to 1989. Br J Obstet Gynaecol. 1991; 98: 550-557.
- Buza N, Rutherford T, Hui P. Genotyping diagnosis of nongestational choriocarcinoma involving fallopian tube and broad ligament: a case study. Int J Gynecol Pathol. 2014; 33: 58-63.
- Aranake-Chrisinger J, Huettner PC, Hagemann AR, Pfeifer JD. Use of short tandem repeat analysis in unusual presentations of trophoblastic tumors and their mimics. Hum Pathol. 2016; 52: 92-100.
- Cankovic M, Gaba AR, Meier F, Kim W, Zarbo RJ. Detection of nonmaternal components of gestational choriocarcinoma by PCR-based microsatellite DNA assay. Gynecol Oncol. 2006; 103: 614-617.