Case Report
Ganglioneuroblastoma of the Nodular Variant in an Adult Female: Diagnostic and Treatment Dilemmas
Rachel A Alter1, Marjan Alimi1, Todd Anderson2, Christopher Filippi2, David Langer1,2, Richard S Lazzaro5, Mitchell E Levine1,2, Bidyut Pramanik2, Stephen Scharf2, Julia R Schneider1 and John A Boockvar1,2,4*
1Lenox Hill Brain Tumor Center, Lenox Hill Hospital, USA
2Departments of Neurosurgery, Neurology, Radiology, and Pathology, Hofstra Northwell School of Medicine, USA
3Departments of Neurosurgery and Radiology, Weill Cornell Medical College of Cornell University, USA
4Department of Neurological Surgery, Hofstra Northwell School of Medicine, USA
5Departments of Cardiothoracic Surgery, Lenox Hill Hospital, USA
*Corresponding author: John A Boockvar, Department of Neurosurgery, Hofstra Northwell School of Medicine, 130 East 77th Street, 3rd Floor, Black Hall Building, New York, NY 10075, USA
Published: 31 Oct, 2016
Cite this article as: Alter RA, Alimi M, Anderson T, Filippi
C, Langer D, Lazzaro RS, et al.
Ganglioneuroblastoma of the Nodular
Variant in an Adult Female: Diagnostic
and Treatment Dilemmas. Ann Clin
Case Rep. 2016; 1: 1169.
Abstract
Ganglioneuroblastoma (GNB) is a common pediatric cancer but rarely presents in adults. We describe a case of a 25-year-old woman who was diagnosed with GNB nodular (GNBn) after presenting with severe back pain. Our patient underwent a gross total resection and is currently under observation with no further treatment. Available treatment options are discussed in the case of recurrence.
Keywords: Ganglioneuroblastoma; Nodular variant; Neuroblastoma in adults
Introduction
Neuroblastomas are heterogeneous tumors with a potential for varied differentiation and can
spontaneously regress, become malignant, or act benign [1]. They derive from neural crest cells
[2] and therefore can develop into any organ in the sympathetic nervous system [3]. Neuroblastic
tumors are most commonly found in pediatric patients, and when they are diagnosed in adults, they
are classified as high-risk tumors associated with a poor prognosis [4].
Neuroblastic tumors can present in three ways, from least differentiated to most highly
differentiated: neuroblastoma (NB), ganglioneuroblastoma (GNB), and ganglioneuroma (GN) [5].
The patient in this case study presented with GNB, a rare tumor that occurs almost exclusively
in children. Roughly 650 cases in the United States are reported annually, 50% of which occur in
children under the age of 2, and 75-85% of which occur in children under the age of 4 [6]. While it
is one of the common pediatric cancers, fewer than 50 cases of adult GNB have been reported [7].
Prognosis in adults depends on the extent of resection, with gross total resections (R0) having the
most favorable outcome [8].
Though malignant, GNB is less aggressive than NB, and consists of small, round, immature
neuroblast cells and mature ganglion cells [9]. GNB can be further divided into two subtypes,
including intermixed and nodular [10]. Intermixed GNBs consist of microscopic nests of
neuroblastoma situated in a ganglioneuromatous stroma [7]. Nodular GNBs contain gross nodulesimmature
small cells-of neuroblastoma situated in large expanses of ganglioneuroma-big, mature
cells in a fusiform stroma [7]. Our patient presented with GNB, nodular (GNBn).
Case Presentation
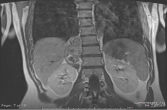
A 25 year-old female presented with severe right-sided back pain in March 2016. A CT scan
from March 2016 revealed a right paraspinal mass with curvilinear calcifications depicted in
(Figure 1). She was sent for an MRI, which revealed a heterogeneous enhancement with three
nodular components. Several differential diagnoses were initially considered, including plexiform
schwannoma, sarcoma, and metastasis. The diagnosis was confirmed upon core-needle biopsy of the
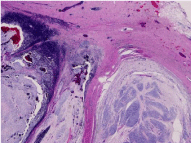
right paraspinal soft tissue, which revealed a biphasic mass as shown in (Figure 2). One component
consisted of bland spindle cells and a few admixed cells with features of ganglion cells. The second
component was composed of atypical epithelioid cells with increased mitotic activity, focal
rhabdoid cells, and multinucleated cells. Immunohistochemistry (IHC) revealed findings consistent with ganglioneuroblastoma (GNB), highly likely the nodular variant
(GNBn) (Figure 3 and 4). The tissue was negative for MYCN gene
amplification, suggesting a less aggressive tumor and better prognosis
for the patient [11].
Prior to surgery, the patient was sent for an MIBG scan, an
imaging test commonly used in lieu of PET to confirm up take of
radioisotopes by foci of neuroendocrine tumor cells. A gross total
resection of the spinal tumor with neurosurgery and thoracic surgery
was performed in April 2016. Tumor margins were negative. Tissue
was sent to Foundation One for next-generation genetic sequencing,
which revealed an ALK point mutation at R1275Q. ALK encodes a
receptor tyrosine kinase (RTK) that is part of the insulin receptor
super family and induces downstream activation of pathways
associated with cell survival, angiogenesis, and proliferation [12].
ALK mutations in neuroblastoma are associated with increased ALK
protein expression and shorter survival [13-15]. After gross total
resection, observation alone was decided for the patient. Currently,
she is stable and receiving no radiation or chemotherapy, as her
imagery findings continue to show no residual cancer.
Figure 1
Figure 1
Coronal T2 MRI image demonstrates 3 nodular components on the
right lumbar spinal region with mixed T2 signal.
Figure 2
Figure 2
Biphasic appearance consisting of well-circumscribed hypercellular
nodules in a background of hypocellular stroma.
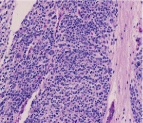
Figure 3
Figure 3
Higher power image of nodules. Consist of blue cells with high
nuclear/cytoplasmic ratio, fine chromatin, and low mitotic/karyorrhectic index.
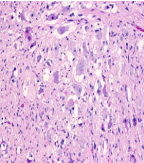
Figure 4
Figure 4
High power image of hypocellular areas show bland spindle cells
with admixed ganglion cells.
Discussion
We present a case of nodular GNB in a 25-year-old female to
highlight some of the difficulties in recommending treatment for
this type of tumor. GNB is a rare disease that almost exclusively
affects pediatric population; fewer than 50 cases have been reported
in adults. In all cases, the treatment of choice was radical resection
[9]. According to one study, every adult patient whose tumor was
only partially resected or not resected died within 24 months [16]. Recurrence has been found to occur most frequently within the first
two years after surgery [9]. Therefore, evidence supports the high
importance of close clinical follow up, as well as imaging including
CT scan and MIBG scintigraphy every 6 months, within the first two
years after surgery [16]. Additionally, patients with GNBn have been
found to have a high risk for distant metastases [17].
As for chemotherapy options, given that there have only been a
few cases of adult GNB to set treatment precedence, we looked to
pediatric cases for guidance. Active chemotherapeutic agents currently
in use for pediatric population include cyclophosphamide vincristine,
adriamycin, and combinations with platinum and etoposide. In the
event of recurrence, topotecan [18] and temozolomide [19] have
been demonstrated effective in the pediatric population. Additional
drugs have been suggested based on the patient’s particular genetic
alterations. Crizotinib is an inhibitor of MET, ALK, RO1, and RON
kinases and is FDA-approved to treat patients with metastatic nonsmall
cell lung cancer (NSCLC) whose tumors are positive for ALK or
ROS1 rearrangements [20]. Similarly, ceritinib inhibits ALK, ROS1,
IR, and IGF-IR kinases and is approved for patients with NSCLC in
patients whose tumors are positive for ALK rearrangements and who
are intolerant to crizotinib [20-23]. Alectinib is another option that
has been approved for NSCLC for patients who have progressed or
are intolerant to crizotinib [20].
Conclusion
The current study provides additional evidence for the importance of a gross total resection in treatment of GNB in adults. Additionally, this case study presents several treatment options based on pediatric precedents and the patient’s unique genetics. Close post-operative follow up increases the chance for early detection in the cases of recurrence and higher chance for survival.
Consent
The patient has consented to the use of their health information for research purposes.
Conflict of Interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
References
- Lonergan GJ, Schwab CM, Suarez ES, Carlson CL. Neuroblastoma, ganglioneuroblastoma, and ganglioneuroma: radiologic-pathologic correlation. Radiographics. 2002; 22: 911-934.
- Davidoff AM. Neuroblastoma. Semin in Pediatr Surg. 2012; 21: 2-14.
- Franks LM, Bollen A, Seeger RC, Stram DO, Matthay KK. Neuroblastoma in adults and adolescents: An indolent course with poor survival. Cancer. 1997; 79: 2028-2035.
- Jrebi NY, Iqbal CW, Joliat G, Sebo TJ, Farley DR. Review of our experience with neuroblastoma and ganglioneuroblastoma in adults. World J Surg. 2014; 38: 2871-2874.
- Shimada H, Umehara S, Monobe Y, Hachitanda Y, Nakagawa A, Goto S, et al. International neuroblastoma pathology classification for prognostic evaluation of patients with peripheral neuroblastic tumors: a report from the Children’s Cancer Group. Cancer. 2001; 92: 2451-2461.
- Perrino C. Adrenal gland and paraganglia: Neuroblastic tumors: Ganglioneuroma. 2016.
- Peycru T, Guiramand J, Tardat E, Savoie PH, Avaro JP, Balandraud P. Nodular ganglioneuroblastoma in adults. Can J Surg. 2009; 52: 4.
- Koike K, Iihara M, Kanbe M, Omi Y, Aiba M, Obara T. Adult-type ganglioneuroblastoma in the adrenal gland treated by laparoscopic resection: report of a case. Surg Today. 2003; 33: 785-790.
- Bolzacchini E, Martinelli B, Pinotti G. Adult onset of ganglioneuroblastoma of the adrenal gland: case report and review of the literature. Surg Case Rep. 2015; 1: 79.
- Weiss S, Enzinger J. Soft tissue tumors. 4th ed. St. Louis (MO): Mosby; 2003.
- Valentijn LJ, Koster J, Haneveld F, Aissa RA, van Sluis P, Broekmans ME, et al. Functional MYCN signature predicts outcome of neuroblastoma irrespective of MYCN amplification. Proc Natl Acad Sci U S A. 2012; 109: 19190-19195.
- Grande E, Bolos MV, Arriola E. Targeting oncogenic ALK; a promising strategy for cancer treatment. Mol Cancer Ther. 2011; 10: 569-579.
- Bresler SC, Weiser DA, Huwe PJ, Park JH, Krytska K, Ryles H, et al. ALK mutations confer differential oncogenic activation and sensitivity to ALK inhibition therapy in neuroblastoma. Cancer Cell. 2014; 26: 682-694.
- Bellini A, Bernard V, Leroy Q, Rio Frio T, Pierron G, Combaret V, et al. Deep sequencing reveals occurrence of subclonal ALK mutations in neuroblastoma at diagnosis. Clin Cancer Res. 2015; 21: 4913-4921.
- Duijkers FA, Gaal J, Meijerink JP, Admiraal P, Pieters R, de Krijger RR, et al. High analplastic lymphoma kinase immunohistochemical staining in neuroblastoma and ganglioneuroblastoma is an independent predictor of poor outcome. Am J Pathol. 2012; 180: 1223-1231.
- Caron HN, Pearson ADJ. Neuroblastoma. In: Voute PA, Barrett A, Stevens MCG, Caron HN, editors. Cancer in children. 5th ed. Oxford: Oxford University Press; 2005; 337-352.
- Umehara S, Nakagawa A, Matthay KK, Lukens JN, Seeger RC, Stram DO, et al. Histopathology defines prognostic subsets of ganglioneuroblastoma, nodular. American Cancer Society. 2000; 89: 1150-1161.
- Garaventa A, Luksch R, Biasotti S, Severi G, Pizzitola MR, Viscardi E, et al. A phase II study of topotecan with vincristine and doxorubicin in children with recurrent/refractory neuroblastoma. Cancer. 2003; 98: 2488-2494.
- Rubie H, Chisholm J, Defachelles AS, Morland B, Munzer C, Valteau- Couanet D, et al. Phase II study of temozolomide in relapsed or refractory high-risk neuroblastoma: a joint SocieteFrancaise des Cancers de l’Enfant and United Kingdom Children Cancer Study Group-New Agents Group Study. J Clin Oncol. 2006; 24: 5259-5264.
- Foundation One Genetics Report. 2016.
- Clynes D, Higgs DR, Gibbons RJ. The chromatin remodeller ATRX: a repeat offender in human disease. Trends Biochem Sci. 2013; 38: 461-466.
- Ratnakumar K, Bernstein E. ATRX: the case of a peculiar chromatin remodeler. Epigeneitcs. 2013; 8: 3-9.
- Kohler JA, Rubie H, Castel V, Beiske K, Holmes K, Gambini C, et al. Treatment of children over the age of one year with unresectable localized neuroblastoma without MYCN amplification: results of the SIOPEN study. Eur J Cancer. 2013; 49: 3671-3679.