Case Report
Focal Fibrocartilaginous Dysplasia (FFCD)
Saritha Ranabothu1*, Daniel Schwartz2 and Marcela Del Rio1
1Department of Pediatric Nephrology, Montefiore Medical Center, USA
2Department of Pathology, Montefiore Medical Center, USA
*Corresponding author: Saritha Ranabothu, Department of Pediatric Nephrology, Children’s Hospital at Montefiore, Albert Einstein College of Medicine, Montefiore Medical Center, 111 East 210th Street, Bronx, NY, 10467, USA
Published: 24 Oct, 2016
Cite this article as: Ranabothu S, Schwartz D, Rio MD.
Early Diabetic Nephropathy in a
Pediatric Renal Transplant Recipient
Leading to End Stage Renal Disease.
Ann Clin Case Rep. 2016; 1: 1163.
Abstract
Limited data is available on post-transplant diabetes mellitus (PTDM) and diabetic nephropathy in the pediatric population. Annual prevalence of diabetes and diabetic nephropathy in the general pediatric population is on the rise. In the non- transplant pediatric population, diabetic nephropathy occurs 10-15 years after the diagnosis of diabetes mellitus (DM). As per our knowledge, this is the first reported pediatric case of diabetic nephropathy that occurred within 3 years of the diagnosis of PTDM, and also developed end stage renal disease within 7 years. Prognosis was worse due to poor compliance with the medication, antibody-mediated rejection and diabetic nephropathy leading to end stage renal disease (ESRD). Patient is currently on dialysis.
Keywords: Diabetes mellitus; Chronic kidney disease; Glomerulo nephritis
Abbreviations
DM: Diabetes Mellitus; PTDM: Post Transplant Diabetes Mellitus; ESRD: End Stage Renal Disease; CKD: Chronic Kidney Disease; MPGN: Membrane Proliferative Glomerulo Nephritis; FSGS: Focal Segmental Glomerulo Sclerosis; HbA1C: Hemoglobin A1C
Case Presentation
21-year old Hispanic female with end stage renal disease (ESRD) due to membrane proliferative
glomerulo nephritis type 2 (MPGN). At 14 years of age, she received a deceased donor renal
transplant. The donor was a healthy 17 year old female who died from a gunshot wound. Donor
kidney biopsy was not performed. Pre-transplant period was significant for gluco corticoidinduced
insulin-dependent diabetes mellitus that resolved after discontinuation of the steroids.
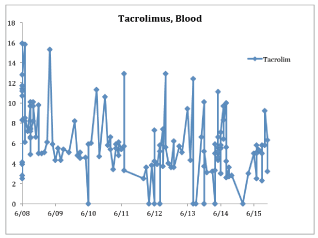
One month post-transplant, she developed gluco corticoid- and tacrolimus- induced type 1 diabetes
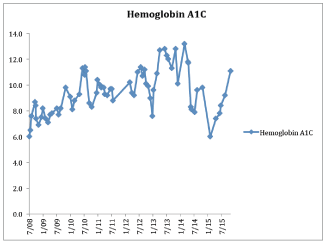
mellitus, poorly controlled (Hemoglobin A1C averaged 10%). Three years post-transplant, patient
developed nephrotic-range proteinuria and microscopic hematuria. Serum complements were
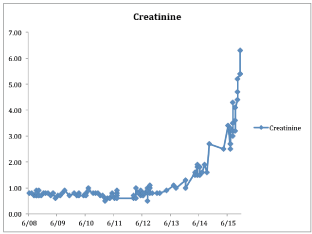
normal suggesting non-recurrence of the MPGN, and the serum creatinine was stable at 0.8mg/
dL. (Laboratory results, Graphs 1-3 and Tables 1 and 2). Patient was started on enalapril with little improvement in the proteinuria. Patient also had persistent
hypertension that was controlled with amlodipine and enalapril.
Fundoscopic exam showed no evidence of diabetic retinopathy. A
graft biopsy was performed to rule out MPGN type 2 recurrence, de
novo glomerulopathy, diabetic nephropathy, or rejection. The biopsy
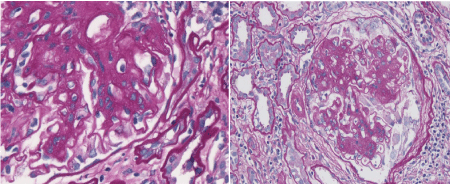
showed basement membrane thickening and mesangial expansion
with focal nodularity, consistent with de novo diabetic nephropathy.
There was isolated mesangial complement 3 (C3) staining that
was non-specific for MPGN. There were no signs of rejection or de
novo glomerulopathy. BK virus staining was negative. Patient
continued to have poorly controlled DM and non-adherence to the
medications. The graft function deteriorated and a repeat biopsy
showed worsening of the diabetic nephropathy, secondary focal
segmental glomerulo sclerosis (FSGS), and chronic antibodymediated
rejection (Figures 1A and B,2,3,4A,B,C and D) with
transplant glomerulopathy. Patient progressed to end-stage renal
disease and hemodialysis was initiated.
The risk factors for the development of DM in this patient
included a maternal history of DM, pre-transplant gluco corticoidinduced
DM, and post-transplant gluco corticoid- and tacrolimusinduced
DM. Her poor compliance with the insulin regimen resulted
in early progression to diabetic nephropathy leading to end stage
renal disease.
Graph 1
Graph 2
Graph 3
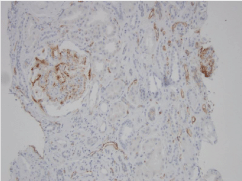
Figure 1
Figure 1
(A) Mesangial expansion and capillary double contours. (B)
Nodular mesangial expansion and arteriolar hyalinosis - PAS.
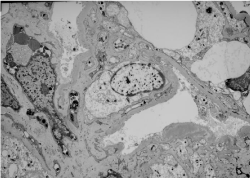
Figure 2
Figure 3
Figure 4
Figure 4
(A) Renal biopsy: Immunofluorescence microscopy showing trace positive for C1q in the mesangium. (B) Renal biopsy: Immunofluorescence microscopy
showing 2+ granular staining in the mesangium. (C) Renal biopsy: Immunofluorescence microscopy showing 1+ granular staining for IgM in the mesangium. (D)
Renal biopsy: Immunofluorescence microscopy showing focal 1(+) granular staining for lambda light chain in capillary walls, predominantly at the periphery of the
glomerular tuft.
Table 1
Table 2
Discussion
The risk of PTDM is well defined in adults but much less understood
in pediatric population. In the adult population, the incidence of de
novo PTDM varies between 2% and 53%, of which 4-25% are renal
transplant recipients [1]. Based on previous studies, 2–35% of
children develop PTDM after renal transplantation [2-4].
Incidence of DM is higher in transplant patients compared to the general population. Adult risk factors associated with the development
of PTDM are age >40 years, obesity, metabolic syndrome, family
history of diabetes mellitus, cadaveric graft, and Afro-American or
Hispanic race. Hepatitis C infection and the use of glucocorticoids,
tacrolimus and sirolimus are risk factors as well [1,5,6]. Pediatric
studies have shown that the risk factors for PTDM are older age,
obesity (Body Mass Index BMI ≥30) and cytomegalovirus (CMV)
naïve recipients who receive a CMV positive graft. Small studies
have shown that a family history of diabetes and peri- transplant
hyperglycemia are also risk factors for the development of PTDM [2].
The occurrence of PTDM is usually within the first six months
following transplantation and is generally reversible [7]. In the nontransplant
pediatric population, diabetic nephropathy usually
develops within 10-15 years after the diagnosis of DM. In the pediatric
population, it is uncommon to develop diabetic nephropathy
within 4-5 years of the diagnosis of DM [8,9]. The development of
PTDM is a major clinical concern following kidney transplantation
and has been shown to be strongly associated with reduced graft
function, increased cardiovascular morbidity and lower patient
survival among adult recipients [4,9-11]. In the pediatric population,
few small studies have shown no direct significant association of
PTDM with graft dysfunction or patient mortality. There is scarce
data available on PTDM in pediatric population leading to graft loss
[4].
Calcineurin inhibitors lead to DM by decreasing glucose uptake,
reducing insulin release or reducing insulin gene expression. Among
calcineurin inhibitors, tacrolimus is more diabetogenic by 30-
50% compared to cyclosporine and its effect is not dose dependent.
Tacrolimus is still the preferred immunosuppressant drug due to
its superior efficacy and safety despite the risk of diabetes [12-14].
Glucocorticoids cause DM by increasing gluconeogenesis, increasing
insulin resistance, reducing insulin release, or depressing beta cell
function.
The choice of immunosuppressant and the obesity are the
only modifiable risk factors reaching significance. Corticosteroids
dose reduction has been shown to significantly improve glucose
tolerance during the first year after transplantation. In patients
with PTDM, tacrolimus to cyclosporine conversion therapy has
shown variable results. However, any dose reduction or change of
immunosuppressive regimen should be weighed against the risk of
rejection, patient’s medication tolerance and side effects [14-17].
Post-transplant serum glucose should be monitored on a regular
basis and if abnormal, a glucose tolerance test should be performed.
Based on the transplant center incidence and prevalence of PTDM, it
is reasonable to obtain a monthly fasting blood glucose and HbA1C,
at least for the first few months after transplant. Prompt referral
to the Endocrinology service is essential to instruct the patients
on home glucose monitoring, carbohydrate counting and insulin
administration, if needed. Patients need to be educated on the
importance of glycemic control and lifestyle modification. If there are
signs of proteinuria or graft dysfunction in an adolescent or young
adult patient with PTDM, diabetic nephropathy should be considered
in the differential diagnosis.
References
- Balla A, Chobanian M. New-onset diabetes after transplantation: a review of recent literature. Curr Opin Organ Transplant. 2009; 14: 375-379.
- Greenspan LC, Gitelman SE, Leung MA, Glidden DV, Mathias RS. Increased incidence in post-transplant diabetes mellitus in children: a case-control analysis. Pediatr Nephrol. 2002; 17: 1-5.
- McKee M, Segev D, Wise B, Case B, Neu A, Fivush B, et al. Initial experience with FK506 (tacrolimus) in pediatric renal transplant recipients. J Pediatr Surg. 1997; 32: 688-690.
- Burroughs TE, Swindle JP, Salvalaggio PR, Lentine KL, Takemoto SK, Bunnapradist S, et al. Increasing incidence of new-onset diabetes after transplant among pediatric renal transplant patients. Transplantation. 2009; 88: 367-373.
- Bastos MA, Oliveira MM, de Castro SH, Cunha EF, Moraes ER, Ruzzani F, et al. [Risk factors for developing diabetes mellitus after renal transplantation]. Arq Bras Endocrinol Metabol. 2005; 49: 271-277.
- Al-Uzri A, Stablein DM, A Cohn R. Posttransplant diabetes mellitus in pediatric renal transplant recipients: a report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS). Transplantation. 2001; 72: 1020-1024.
- Montori VM, Basu A, Erwin PJ, Velosa JA, Gabriel SE, Kudva YC. Posttransplantation diabetes: a systematic review of the literature. Diabetes Care. 2002; 25: 583-592.
- Danne T, Kordonouri O. Controversies on the pathogenesis of diabetic angiopathy: which treatment for normotensive adolescents with microalbuminuria and type 1 diabetes?. J Pediatr Endocrinol Metab. 1998; 11: 347-363.
- Sochett E, Daneman D. Early diabetes-related complications in children and adolescents with type 1 diabetes. Implications for screening and intervention. Endocrinol Metab Clin North Am. 1999; 28: 865-882.
- Wilkinson A, Davidson J, Dotta F, Home PD, Keown P, Kiberd B, et al. Guidelines for the treatment and management of new-onset diabetes after transplantation. Clin Transplant. 2005; 19: 291-298.
- Cosio FG, Pesavento TE, Kim S, Osei K, Henry M, Ferguson RM. Patient survival after renal transplantation: IV. Impact of post-transplant diabetes. Kidney Int. 2002; 62: 1440-1446.
- Bouchta NB, Ghisdal L, Abramowicz D, Broeders N, Surquin M, Hoang AD, et al. Conversion from tacrolimus to cyclosporin is associated with a significant improvement of glucose metabolism in patients with newonset diabetes mellitus after renal transplantation. Transplant Proc. 2005; 37: 1857-1860.
- Radu RG, Fujimoto S, Mukai E, Takehiro M, Shimono D, Nabe K, et al. Tacrolimus suppresses glucose-induced insulin release from pancreatic islets by reducing glucokinase activity. Am J Physiol Endocrinol Metab. 2005; 288: 365-367.
- Araki M, Flechner SM, Ismail HR, Flechner LM, Zhou L, Derweesh IH, et al. Posttransplant diabetes mellitus in kidney transplant recipients receiving calcineurin or mTOR inhibitor drugs. Transplantation. 2006; 81: 335-341.
- Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. 2003; 3: 178- 185.
- Romagnoli J, Citterio F, Nanni G, Favi E, Tondolo V, Spagnoletti G, et al. Incidence of posttransplant diabetes mellitus in kidney transplant recipients immunosuppressed with sirolimus in combination with cyclosporine. Transplant Proc. 2006; 38: 1034-1036.
- Pascual J, van Hooff JP, Salmela K, Lang P, Rigotti P, Budde K. Threeyearobservational follow-up of a multicenter, randomized trial on tacrolimus-basedtherapy with withdrawal of steroids or mycophenolate mofetil after renal transplant. Transplantation. 2006; 82: 55-61.