Case Report
Abdominal Coarctation in a 9 Year Girl Mimicking Anxiety Attacks: Case Report
P Sciacca1*, I Stella2, S Leonardi2, F Sciacca3 and P Guccione4
1Pediatric Neonatologic Cardiovascular Clinic, AOU Policlinico-Vittorio Emanuele, Italy
2Pediatric Bronchopneumology Unit, AOU Policlinico-Vittorio Emanuele, Italy
3Department General Surgery and Medical-Surgical Specialties , University of Catania, Italy
4Pediatric Cardiology Department, Pediatric Hospital Bambin Gesù, Rome
*Corresponding author: P. Sciacca, Pediatric Neonatologic Cardiovascular Clinic, AOU Policlinico- Vittorio Emanuele, via Santa Sofia 78 – 95123 Catania, Italy
Published: 11 Oct, 2016
Cite this article as: Sciacca P, Stella I, Leonardi S, Sciacca
F, Guccione P. Abdominal Coarctation in
a 9 Year Girl Mimicking Anxiety Attacks:
Case Report. Ann Clin Case Rep. 2016;
1: 1156.
Abstract
Background: Abdominal aortic coarctation is an uncommon vascular disease, representing approximately 2% of aortic coarctations, aortic coarctation most commonly occurs in the region
immediately distal to the origin of the left subclavian artery. Clinical presentation of abdominal
localization is variable especially when it occurs in children and may represent a rare cause of
secondary arterial hypertension.
Case Report: We report a case of abdominal aortic coarctation diagnosed precociously in a 9 years
old girl, who had showed non-specific symptoms. She complained dyspnea, retrosternal pain just
before to fall asleep and palpitations and night fears, suggesting a diagnosis of anxiety attacks due
to restarting of school time. We found accidentally increased blood pressure values in one evening
measurement and confirmed by ambulatory blood pressure recording. So we started diagnostic
process for differential diagnosis of hypertension causes and diagnosed abdominal coarctation, first
suggested by an abnormal upper/lower extremities ratio and confirmed by CT and then by Angio-
MRI.
Discussion: Aortic coarctation has a variable clinical presentation, depending on the severity and
the site of obstruction. Abdominal aortic coarctations usually cause signs or symptoms during the
second decade of life; the mean age of diagnosis is 22 years while our patient was just 9 years old.
In our case, clinical presentation was not initially typical for aortic coarctation, because symptoms
might suggest a generic diagnosis of anxiety attacks. Hypertension is not a frequent condition in
children; when it is present, it’s often a secondary form, so it needs a deep differential diagnosis
process.
Conclusion: Abdominal aortic coarctation is a rare pediatric clinical condition, very difficult to
identify because of its rarity and its variable symptoms. Essential was the occasional discovery of
high blood pressure levels confirmed with ambulatory blood pressure monitoring. This element
guided the diagnostic process to the research of causes of hypertension and allowed us to obtain the
correct diagnosis.
Introduction
Abdominal aortic coarctation is a rare localization, the most common site of aortic coarctation
occurs immediately distally to the origin of the left subclavian artery, while abdominal localization
represents quite a an infrequent form that can be on a congenital basis [1], but may also be acquired.
Clinical presentation of abdominal localization is variable and may include arterial hypertension,
heart failure, murmur, claudication, diminished femoral pulses and a leg blood pressure equal to or
lower than arms [2].
We report a rare case of abdominal aortic coarctation diagnosed in a child who had showed
apparently non-specific symptoms: anxiety attacks.
Case Presentation
When patient was admitted in our hospital, parents signed consent to the processing of personal data of the children.
A 9 years old girl was referred to our hospital for dyspnea
and retrosternal pain just before to fall asleep. The patient had a
more recent history of recurring similar episodes, characterized by
palpitations and night fears, in addiction to mild chest pain and
short breathing difficulty; these symptoms should have suggested
to run cardiac clinical and instrumental examinations but remote
pathological anamnesis was characterized by transient patent ductus
arteriosus with six-monthly cardiologic follow-up until the age of 5
years. For this reason only electrocardiogram was performed and a
generic diagnosis of anxiety attacks due to restarting of school time
had been the first diagnosis. Parents decided to ask a second opinion.
On admission, her general conditions were good. Heart
auscultation noted a 1/6 systolic murmur at base of heart. Laboratory
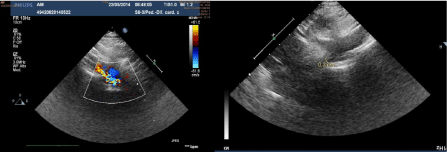
exams, spirometry, electrocardiogram and echocardiography were
normal. During the first day of hospitalization we found an increased
arterial blood pressure value in the evening and for this reason an
ambulatory blood pressure recording was performed that finally
revealed a before now undiagnosed hypertension. We completed
diagnostic process for differential diagnosis of hypertension causes.
The exams showed a normal renal function, a regular circadian rhythm
of plasmatic cortisol levels and normal aldosterone blood levels;
higher renin levels were found in clinostatism and in orthostatism
(respectively 47,10 uUI/ml and 125,40 uUI/ml). In the while we
assessed an abnormal difference in blood pressures between the upper
and lower extremities: arterial blood pressure was 120/84 mmHg at
right arm and 90/73 mmHg at right leg. A new Echocardiography
excluded isthmic aortic coarctation; in effect there was a much rarer,
especially in child, abdominal localization of aortic coarctation.
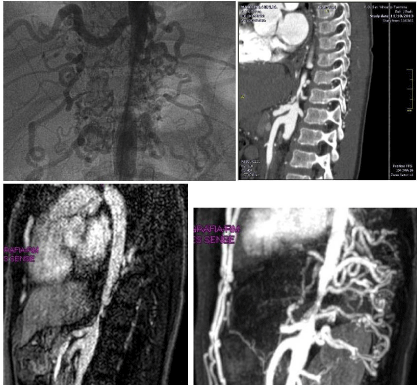
Doppler vascular abdominal echography and contrasted-enhanced
CT of chest and abdomen showed an isolate significant stenosis of
abdominal aorta, with the stenotic tract originating vertebral arteries;
this picture was confirmed by MRI and Angiography (Figure 1 and 2).
Once made the diagnosis, the patient has started a Ca-antagonist
treatment to manage hypertension, while waiting for the choice of
when and which kind of bypass correction.
Figure 1
Figure 2
Discussion
Coarctation of aorta comprises 5% to 10% of congenital
cardiac lesions [2-4] but if we consider only coarctation involving
the abdominal aorta they are much rarer with a rate of 2% of all
coarctations [5].
Abdominal aortic coarctations clinical presentation is variable,
depending on the severity and the site of obstruction. Usually cause signs or symptoms during the second decade of life; the mean age
of diagnosis is 22 years while our patient was just 9 years old. If
untreated, this condition has been associated with stroke, progressive
left ventricular hypertrophy with congestive heart failure and flash
pulmonary edema, and less often with renal insufficiency. In one
review, 55% of untreated patients died at a mean age of 34 years [5].
Symptoms of aortic coarctations are related to the kind and site:
in the neonatal isthmic severe localization there are early symptoms
and signs of heart failure and respiratory distress such as tachypnea,
grunting, retractions, pale skin , heavy sweating, diminished or absent
lower extremity pulses, increased upper extremity pulses, difficulty in
feeding But in the adult post isthmic form patients may remain for
long time almost asymptomatic or may present headache, systemic
hypertension, nose bleeding, muscle weakness, cold feet, leg cramps
and claudication; in effect our patient after diagnosis realized to
have suffered of leg cramps. Physical examination can note strong
upper body pulses and diminished or absent leg pulses, which are
corroborated by a difference in blood pressures between the upper
and lower extremities. After infancy, the systolic blood pressure in
the lower extremities is usually higher (5 to 20 mm Hg) [2-6] than
in the upper extremities as a result of the standing wave effect. If the
leg systolic blood pressure is equal to or lower than the arm pressure,
the diagnosis of aortic coarctation should be considered [2-7], like in
our patient, who presented a systolic pressure gradient of about 30
mmHg in legs.
In our case, clinical presentation and the history of frequent
“normal Echocardiograms” contributed to mislead an early diagnosis
also because specific symptoms began to be evident just before
school restarting suggesting a generic diagnosis of anxiety attacks
that, according to parents’ feelings, conformed with the irritable
disposition of the preadolescent girl. Panic or anxiety disorders may
be characterized by recurrent unexpected episodes of severe anxiety,
which typically reach their peak within 10 minutes and last around
30-45 minutes [8]. These symptoms can look similar to those referred
by our patient, who lamented an anxiety state associated to dyspnea
and heart rate acceleration. Luckily in this case a new complete
extended evaluation of the patient, including blood pressure
ambulatory monitoring, thanks to the individuation of high blood
pressure quickly redirected the diagnosis: in fact hypertension is not a
frequent condition in children with a prevalence ranging from 4% to
17% [9-10]. The probability of a secondary hypertension is inversely
proportional to the age of the patient and directly proportional to
blood pressure levels [11]; but a rare abdominal aortic coarctation
caused just an apparently mild hypertension in this girl.
Regarding abdominal coarctation, we can suppose to have
diagnosed a congenital form. Considering the acquired forms, they
have been associated with pathologies such as Takayasu syndrome,
William’s syndrome, neurofibromatosis, fibromuscular dysplasia,
retroperitoneal fibrosis, and mucopolysaccharidosis [12]. In our
case we could exclude neurofibromatosis, mucopolysaccharidosis
and Williams’s syndrome because of absence of typical stigmata;
so the differential considerations are limited to Takayasu arteritis
and non-inflammatory aorto-arteriopathy, such as fibromuscular
dysplasia [13]. Takayasu arteritis usually is multi distrectual and it
can be differentiated from fibromuscular dysplasia for the general
inflammatory state and by histologic examination [14]. The only
therapeutic differences between the two conditions would be the use
of anti-inflammatory therapy in Takayasu arteritis. D’Souza et al. [14]
with a study in 1998 conclude that differentiating between Takayasu
arteritis and fibromuscular dysplasia may not be as important as the
management itself of aortic coarctation [13].
Management decisions for patients with coarctation of the
aorta depend upon patient age, presentation, and the severity of the
lesion. Medical therapy consists in hypertension management with
antihypertensive drugs (beta-blockers, ACE inhibitors, angiotensinreceptor
blockers or Ca Antagonist), waiting to choose the best
surgical option. There are different methods for the treatment of
aortic coarctation, including surgical repair or percutaneous balloon
angioplasty with or without stent placement [15]. Surgical repair of
coarctation can be achieved by several techniques: resection with
end-to-end anastomosis, subclavian flap aortoplasty in infants
with long-segment coarctation, a bypass graft across the area of
coarctation when the distance to be bridged is too long for an endto-
end repair or prosthetic patch aortoplasty. Balloon angioplasty has been recommended as the preferred treatment for children and adults
with native coarctation or recoarctation after surgery [15]. It has
been suggested that stenting after balloon angioplasty lowers the risk
for complications and has a beneficial effect on long-term survival.
However, primary stenting has an important limitation which is the
failure to adapt to the growing vessel in a child. To overcome this
problem, redilatation during follow-up has been described [16]. In
our case stent implantation can’t be realized because vertebral arteries
originate from the stenotic tract, so we have to exclude this option.
She will need to bypass the stenotic tract with the addition of all the
implications connected with the growth of the girl. In this while the
patient has begun antihypertensive therapy with Ca-antagonists, in
order to manage arterial blood hypertension.
Conclusion
Abdominal aortic coarctation is a rare pediatric clinical condition that can be quite difficult to identify because of its rarity and its variable symptomatology. Anamnesis and physical examination are the first steps to approach any patient. Our case presented initially non-specific clinical features. Essential was the occasional discovery of high blood pressure levels confirmed with ambulatory blood pressure monitoring. This element guided the diagnostic process to the research of causes of hypertension and allowed us to obtain the correct diagnosis.
Author’s Contribution
Conception and design: Pietro Sciacca, Paolo Guccione.
Collection and assembly of data: Ileana Stella, Francesco Sciacca.
Draft of the manuscript: Ileana Stella, Francesco Sciacca.
Critical revision of the manuscript and important intellectual content: Pietro Sciacca, Salvatore Leonardi.
Final approval: Pietro Sciacca.
Acknowledgment
We acknowledge our nurse Maria Mazza to have performed Ambulatory Blood Pressure Monitoring and doctor Giuseppe Belfiore from Pediatric Radiology Department.
References
- Kenneth P, Moresco, Robert S Shapiro. Abdominal aortic coarctation: CT, MRI, and angiographic correlation. Comput Med Imaging Graph. 1995; 19: 427-430.
- Rothman A. Coarctation of the Aorta: an update. Curr Probl Pediatr. 1998; 28: 37-60.
- Fyler DC, Buckley LR, Hellenbrand WE, et al. Report of the New England regional infant cardiac program. Pediatr. 1980; 65: 432-436.
- Tikkanen J, Heinonen OP. Risk factors for coarctation of the aorta. Teratology. 1993; 47: 565-572.
- Stanley J, Criado E, Eliason J, Upchurch GR, Berguer R, Rectenwald Je, et al. Abdominal aortic coarctation: Surgical treatment of 53 patients with a thoraco abdominalby pass, patchaortoplasty, or interposition aortoaorticgraft. J Vasc Surg. 2008; 48: 1073-1082.
- Crapanzano MS, Strong WB, Newman IR, Hixon L, Casal D, Linder CW. Calf blood pressure: clinical implications and correlations with arm blood pressure in infants and young children. Pediatr. 1996; 97: 220-224.
- Park MK, Lee DH, Johnson GA. Oscillometric blood pressures in the arm, thigh, and calf in healthy children and those with aortic coarctation. Pediatr. 1993; 91: 761-765.
- Brandish EK, Baldwin DS. Anxiety disorders. Psychiatric disor. 2012; 40:11.
- Dingman JR, Zaveri PP. Severe Hypertension in a 4-Year-Old Child. Clin pediatric Emerg Med. 2007; 8: 131-136.
- Chiolero A, Bovet P, Paradis G, Paccaud F. Has blood pressure increased in children in response to the obesity epidemic?. Pediatric. 2007; 119: 544- 553.
- Flynn JT, Daniels SR, Hayman LL, Maahs DM, McCrindle BW, Mitsnefes M, et al. Update: Ambulatory Blood Pressure Monitoring in Children and Adolescents: A Scientific Statement From the American Heart Association. Hypertension. 2014; 63: 1116-1135.
- Trimarchi S, Tolva V, Grassi V, Frigiola A, Mario C, Rampoldi V. Descending thoracic and abdominal aortic coarctation in the young: surgical treatment after percutaneous approaches failure. J Vasc Surg. 2008; 47; 4: 865-867.
- Gill DG, Mendes B, Cameron JS, Joseph MC, Chantler C. Analysis of 100 children with severe and persistent hypertension. Arch Dis Child. 1976; 51: 951-956.
- D’Souza S, Tsai WS, Silver MM, Chait P, Benson LN, Silverman E, et al. Diagnosis and management of stenoticaorto-arteriopathy in childhood. J Pediatric. 1998; 132: 1016-1022.
- Jurcut R, Daraban AM, Lorber A, Deleanu D, Amzulescu MS, Zara C, et al. Coarctation of the aorta in adults: what is the best treatment?Case report and literature review. J Med Life. 2011; 4: 189‐195.
- Luijendijk P, Bouma BJ, Groenink M, Boekholdt M, Hazekamp MG, Blom NA, et al. Surgical Versus Percutaneous Treatment of Aortic Coarctation New Standards in an Era of Transcatheter Repair. Expert Rev Cardiovasc Ther. 2012; 10: 1517-1531.