Research Article
Characteristics of Orofacial Amyloidosis: A Case Series
Xiaosong Liu*, Peiru Zhou and Hong Hua
Department of Oral Medicine, Peking University School and Hospital of Stomatology, China
*Corresponding author: Xiaosong Liu, Department of Oral Medicine, Peking University School and Hospital of Stomatology, Beijing 100081, China
Published: 01 Nov, 2016
Cite this article as: Liu X, Zhou P, Hua H. Characteristics of Orofacial Amyloidosis: A Case Series. Ann Clin Case Rep. 2016; 1: 1133.
Abstract
Amyloidosis derived from abnormal extracellular fibril deposits may contribute to multiple organ dysfunctions. The recognition of amyloidosis-associated orofacial changes may be beneficial for early diagnosis. This retrospective study determined the characteristics of orofacial amyloidosis to aid in recognition of this disease. The study included 11 patients who visited Peking University School of Stomatology from 1993 to 2015 and were diagnosed with orofacial amyloidosis. The median age at onset, most commonly affected site, predominant oral feature of amyloidosis, and complications were presented. We concluded that the recognition of amyloidosis-associated orofacial changes may be beneficial for the diagnosis of amyloidosis and the discovery of underlying disease.
Introduction
Amyloidosis is a cluster of heterogeneous diseases caused by the extracellular deposition
of insoluble fibrillar proteins [1]. Amyloidosis is generally classified into three types: primary
amyloidosis, secondary amyloidosis, and familial or hereditary amyloidosis [2]. Based on the site of
fibrillar protein deposition, the disease can also be divided into localized or systemic amyloidosis.
Primary systemic amyloidosis is often attributed to plasma cell dyscrasia arising from multiple
myeloma or other clonal B cell diseases [3]. Secondary (or reactive) systemic amyloidosis is usually
derived from inflammatory diseases such as rheumatoid arthritis, chronic suppuration tuberculosis,
Hodgkin’s lymphoma, syphilis, and rickets [4]. The kidneys, liver, heart, and peripheral nervous
system are affected most often, leading to nonspecific disorders such as proteinuria, liver
enlargement and functional disorders, arrhythmia, heart hypertrophy, and cardiac insufficiency
[5]. Gastrointestinal tract functional disturbances, bleeding, and blockage may also result from
amyloidosis. Ultimately, single- or multiple-organ dysfunction develops. About 80% of the patients
with primary systemic amyloidosis do not survive for 2 years [5].
Although systemic amyloidosis is incurable, an early accurate diagnosis may facilitate treatment
to reduce the amyloid production and prevent the exacerbation of organ dysfunction [5]. The
nonspecific manifestations of amyloidosis make the histopathological findings of amyloid materials
in the affected tissues crucial for the diagnosis. However, the technical difficulty, bleeding, rare
possibility of organ perforation, and patient discomfort limit biopsies of the liver, kidneys, and
heart, which are important visceral organs [6]. Consequently, the British Committee for Standards
in Hematology (BCSH) recommended a less invasive biopsy of an accessible site [7]. The orofacial
region may be the best alternative because of its open nature, lower risk of biopsy-associated
complications, and status as a commonly affected region in amyloidosis [8]. Therefore, the clinical
characteristics of orofacial amyloidosis were summarized here for recognition.
Materials and Methods
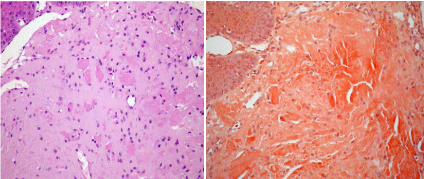
This study included patients who visited the clinic of Peking University School of Stomatology from 1993 to 2015 and were diagnosed with orofacial amyloidosis based on the clinical manifestations and histopathological findings. Histological diagnoses were made by two pathologists, separately, based on the findings of a biopsy taken from the orofacial lesions. Both hematoxylin and eosin (H&E) and Congo red staining showed eosinophilic or orange-pink amorphous homogenous amyloid deposits in the lamina propria (Figure 1). The demographic data, chief complaint, medical history, oral clinical assessment, finding of systemic diseases, and results of laboratory examinations were collected.
Results
During the past 21 years, 11 amyloidosis patients were initially diagnosed in our hospital based on orofacial abnormalities. The median age of onset of these patients was 62 (range 17–74 years)
years, and the male-to-female ratio was 2:3. Before making the diagnosis, the oral clinical signs were present for 0.5–2 years in 81.8% of the patients (9/11). The median
disease duration was 2 years. One subject (subject LXL) had a 13-year
disease history, dating back to the age of 17 years (Table 1).
The tongue was the most common site affected (8/11, 72.7%),
followed by the buccal mucosa (3/11), lip (2/11), gingiva (1/11),
and parotid gland (1/11). Three patients had multiple affected sites
in the orofacial region. The oral mucosal lesions mainly presented
with multiple, painless, waxy, well-circumscribed hard nodules 3–40
mm in diameter. The nodules were accompanied by purple nodules
that did not fade under pressure in subject ZSX. One patient (subject
ZGQ) presented with diffuse, hard enlargement of the tongue
(macroglossia) (Figure 2). Bruising was found in both the labial and
buccal mucosae of subject FP and occurred in the skin of subject YJ.
Bilateral symmetrical enlargement of the parotid glands
was observed in subject DXE, who had been diagnosed with
cryoglobulinemia previously and complained of painful, swollen
parotid glands together with xerostomia and dry eyes. Multiple
small nodules were palpated on the surface of the affected glands,
accompanied by viscous liquid shedding from the gland duct on
pressure. Under ultrasonic examination, the affected parotids
exhibited heterogeneous changes. Amyloidosis was confirmed by
pathologists based on a parotid gland incisional biopsy. A bone marrow smear was performed due to amyloidosis and cryoglobulinemia, both
of which are associated with multiple myeloma, although no evidence
of multiple myeloma was observed in this case.
Two patients had multiple myeloma as a complication. In subject
YJ, the oral mucosal lesions occurred 1.5 years after the diagnosis
of multiple myeloma and were restricted to the tongue. The other
patient (subject FP) had a 30-year medical history of psoriasis with
lesions in the labial, buccal, and lingual mucosae as well as the chest
skin, which occurred 2 years before the multiple myeloma developed.
Subject FP developed osteoporosis and subject YJ progressed with
osteolysis (Table 1).
Figure 1
Figure 1
a) Photomicrograph of the lesion showing the surface epithelium
and homogenous eosinophilic area in the connective tissue (H&E, 40×). b)
Photomicrograph of the lesion showing the surface epithelium; the amyloid
appears orange-pink in the connective tissue (Congo red, 40×). (The
photomicrographs were taken from the pathological slices from subject YJ.)
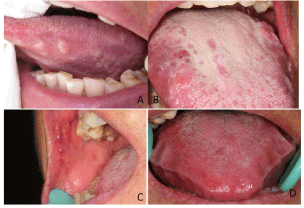
Figure 2
Figure 2
a) Intraoral photograph showing multiple, painless, waxy, wellcircumscribed
hard nodules involving the lateral side of the tongue in
subject YJ. b) Intraoral photograph showing multiple, painless, waxy, wellcircumscribed
hard nodules accompanied by purple nodules without fading
under pressure involving the tongue in subject ZSX. c) Intraoral photograph
showing the lesion involving the buccal mucosa in subject ZSX. d) Intraoral
photograph showing diffuse, hard enlargement of tongue (macroglossia) in
subject ZGQ.
Discussion
Amyloidosis is a rare disease mainly involving older populations
[9,10] of systemic amyloidosis patients, 90% will develop amyloid
deposits in the head, neck, or respiratory tract [11], and a previous
study indicated that 65–70% of adults visit a dental clinic at least once
a year [12]. Therefore, amyloidosis-associated oral mucosal changes
may be recognized initially by oral health professionals, and this is
beneficial for the discovery of underlying diseases. The results of this
study indicate that about 82% of the included patients developing
orofacial amyloidosis were around the age of 60 years, although one
patient was diagnosed at 17 years of age and the orofacial abnormality
had persisted for 13 years. The major disease duration was 0.5 to 2
years. These observations suggest that orofacial amyloidosis is usually
asymptomatic and insidious over a long period. A lack of recognition
of the disease leads to neglect by clinicians; in many cases, it is not
discovered until autopsy [13].
The tongue was the most frequently affected site in this study,
followed by the buccal and labial mucosae, gingiva, and parotid
gland. Multiple, painless, waxy nodules were the predominant
manifestations on the oral mucosa. One patient also exhibited oral
purple nodules, which were the same as those reported by Babburi
et al. [14]. Two patients had mucocutaneous ecchymosis, which was
thought to be associated with multiple myeloma [15]. Macroglossia
was found occasionally, although it is generally considered to be most
common in the oral cavity of systemic amyloidosis patients [11,15].
Amyloid infiltration in the major salivary glands is rare, and
may be localized or secondary to systemic amyloidosis, presenting
with even or lobulated gland enlargement [16-18]. In this study,
enlargement of the parotid gland was seen with multiple nodulelike
lesions. The patient with parotid gland amyloidosis had a
high serum level of rheumatoid factor (RF) and suffered from
concomitant cryoglobulinemia. Cryoglobulinemia is characterized
by the presence of cryoglobulins in the serum at low temperatures.
The marked increase in RF in subject DXE may be ascribed to the
deposition of cryoglobulins [19], of which types II and III have RF
activity [20]. Both amyloid and cryoglobulins may be derived from
lymphoproliferative disorders such as multiple myeloma [21,22].
In this regard, lymphoma resulting from monoclonal lymphocyte
proliferation should be excluded. No histopathological evidence was
found in the present study for lymphoproliferative disorders.
Primary systemic amyloidosis may arise from multiple myeloma
or other clonal B cell diseases [3]. Approximately 15–20% of primary
systemic amyloidosis patients has multiple myeloma and vice versa
[12]. Amyloidosis may develop before or after multiple myeloma, and
physicians should be aware of the close relationship between these
disorders. Osteoporosis and even osteolysis may occur secondary to multiple myeloma, as observed in the present study. With the
expansion of neoplastic plasma cells within the bone marrow
in multiple myeloma, normal bone homeostasis maintained by
activated osteoblasts and osteoclasts is disrupted. Osteoclast activity
is promoted by proteins secreted from stromal cells, while osteoblast
activity is inhibited [23,24]. Primary cutaneous nodular amyloidosis
was histologically considered to be identical to myeloma-associated
systemic amyloidosis with monoclonal immunoglobulin light chain
deposits, and may be complicated with psoriasis [25]. Similarly,
both psoriasis and cutaneous amyloidosis developed in one patient
with multiple myeloma (subject FP). This suggests that patients with
monoclonal immunoglobulin light chain-associated amyloidosis are
susceptible to psoriasis.
In summary, orofacial amyloidosis may present with macroglossia
and diffuse lingual enlargement or with asymptomatic multiple,
waxy, or purple nodules on the lingual and buccal mucosae, gingiva,
and parotid gland. It may occur secondary to multiple myeloma
or be complicated with cryoglobulinemia, psoriasis, osteoporosis,
osteolysis, or other nonspecific disorders of the liver, kidneys,
heart, and neurological system. The recognition of amyloidosisassociated
orofacial changes and relevant systemic diseases by oral
clinicians may be of benefit in the diagnosis of amyloidosis and the
discovery of underlying diseases; moreover, it may limit further
disease progression. However, the high frequencies of nonspecific
complications in older populations, together with the limitations of
the retrospective nature of this study, make it difficult to clarify the
characteristics of orofacial amyloidosis further.
References
- Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003; 349; 583-596.
- Bucci T, Bucci E, Rullan AM, Bucci P, Nuzzolo P. Localized amyloidosis of the upper gingiva: A case report. J Med Case Rep. 2014; 8: 198.
- Pepys MB. Amyloidosis. Annu Rev Med. 2016; 57: 223-241.
- Li Y, Liu N, Xu Y, Wang J, Wu L, Zhou Y, et al. Widespread purple bullalike masses of the oral mucosa. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012; 114: 552-557.
- Gillmore JD, Wechalekar A, Bird J, Cavenagh J, Hawkins S, Kazmi M, et al. Guidelines on the diagnosis and investigation of Al amyloidosis. Br J Haematol. 2015; 168: 207-218.
- Mollee P, Renaut P, Gottlieb D, Goodman H. How to diagnose amyloidosis. Intern Med J. 2014; 44: 7-17.
- Guidelines Working Group of UK Myeloma Forum, British Committee for Standards in Haematology. Guidelines on the diagnosis and management of Al amyloidosis. Br J Haematol. 2004; 125: 681-700.
- Andreadis D, Poulopoulos A, Papadopoulos P, Epivatianos A. Localized tongue amyloidosis in a patient with neurofibromatosis type II. Head Neck Pathol. 2011; 5: 302-305.
- Pinney JH, Smith CJ, Taube JB, Lachmann HJ, Venner CP, Gibbs SD, et al. Systemic amyloidosis in England: An Epidemiological Study. Br J Haematol. 2013; 161: 525-532.
- Real de Asúa D, Costa R, Contreras MM, Gutiérrez Á, Filigghedu MT, Armas M. Clinical characteristics of the patients with systemic amyloidosis in 2000-2010. Rev Clin Esp (Barc). 2013; 213: 186-193.
- Lebowitz RA, Morris L. Plasma cell dyscrasias and amyloidosis. Otolaryngol Clin North Am. 2003; 36: 747-764.
- Greenberg BL, Glick M. Assessing systemic disease risk in a dental setting: A public health perspective. Dent Clin North Am. 2012; 56: 863-874.
- Picken MM. Modern approaches to the treatment of amyloidosis: The critical importance of early detection in surgical pathology. Adv Anat Pathol. 2013; 20: 424-439.
- Babburi S, B Ramya, Rv Subramanyam, V Aparna, Srivastava G. Amyloidosis of the tongue-report of a rare case. J Clin Diagn Res. 2013; 7: 3094-3095.
- Kyle R. Plasma cell disorders. In: Goldman L, Bennett JC, ed. Cecil’s Textbook of Medicine. 21st ed. Philadelphia: WB Saunders; 2000. 985-987.
- Finkel KJ, Kolansky DM, Giorgadze T, Thaler E. Amyloid infiltration of the salivary glands in the setting of primary systemic amyloidosis without multiple myeloma. Otolaryngol Head Neck Surg. 2006; 135: 471-472.
- Kurokawa H, Takuma C, Tokudome S, Yamashita Y, Kajiyama M. Primary localization amyloidosis of the sublingual gland. Fukuoka Igaku Zasshi. 1998; 89: 216-220.
- Kikuta S, Takeda H, Kumakawa K, Yamane M. A case of primary amyloidosis of salivary glands with bronchial amyloidosis. Practica Oto- Rhino-Laryngologica. 2003; 96: 609-613.
- Bournia VK, Vlachoyiannopoulos PG. Subgroups of Sjögren syndrome patients according to serological profiles. J Autoimmun. 2012; 39: 15-26.
- Damoiseaux J, Cohen Tervaert JW. Diagnostics and treatment of cryoglobulinaemia: It takes two to tango. Clin Rev Allergy Immunol.2014; 47: 299-310.
- Rieu V, Cohen P, André MH, Mouthon L, Godmer P, Jarrousse B, et al. Characteristics and outcome of 49 patients with symptomatic cryoglobulinaemia. Rheumatology (Oxford). 2002; 41: 290-300.
- Ninomiya S, Fukuno K, Kanemura N, Goto N, Kasahara S, Yamada T, et al. IgG Type multiple myeloma and concurrent IgA type monoclonal gammopathy of undetermined significance complicated by necrotizing skin ulcers due to type I cryoglobulinemia. J Clin Exp Hematop. 2010; 50: 71-74.
- Giuliani N, Bataille R, Mancini C, Lazzaretti M, Barillé S. Myeloma cells induce imbalance in the osteoprotegerin/osteoprotegerin ligand system in the human bone marrow environment. Blood. 2001; 98: 3527-3533.
- Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, et al. The role of the Wnt-signaling antagonist Dkk1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003; 349: 2483-2494.
- Ung CY, Carr NJ, Ardern-Jones MR. Primary cutaneous nodular amyloidosis associated with psoriasis. Clin Exp Dermatol. 2014; 39: 608- 611.