Case Report
HBV Reactivation in a Non-Hodgkin Lymphoma Patient with Resolved HBV Receiving Rituximab Maintenance Therapy
Chen YT1, Chang CH2, Yeh HZ2,3, Chang CS2,4 and Yang SS2,3*
1Division of Gastroenterology & Hepatology, Feng Yuan Hospital of the Ministry of Health & Welfare, Taichung, Taiwan
2Division of Gastroenterology & Hepatology, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung, Taiwan
3Faculty of Medicine, National Yang-Ming University, Taipei, Taiwan
4Department of Medicine, Chung-Shan Medical University, Taichung, Taiwan
*Corresponding author: Yang SS, Division of Gastroenterology & Hepatology, Department of Internal Medicine, Taichung Veterans General Hospital, 1650 Taiwan Boulevard Sec. 4, Taichung, Taiwan
Published: 12 Sep, 2016
Cite this article as: Chen YT, Chang CH, Yeh HZ, Chang
CS, Yang SS. HBV Reactivation in a
Non-Hodgkin Lymphoma Patient with
Resolved HBV Receiving Rituximab
Maintenance Therapy. Ann Clin Case
Rep. 2016; 1: 1130.
Abstract
Introduction: Rituximab induced hepatitis B virus (HBV) reactivation in both HBsAg-positive
and HBsAg-negative chronic hepatitis B patients had been reported. Among these studies, most
rituximab therapy was concomitant with chemotherapy including steroid. Whether rituximab
maintenance monotherapy could induce HBV reactivation and resulting in serious complications
is unknown.
Case Presentation and Conclusion: We report one patient who has negative hepatitis B surface
antigen (HBsAg-negative) and positive antibodies to hepatitis B surface antigen (anti-HBs-positive)
prior to rituximab-containing chemotherapies develops HBV reactivation followed by fulminant
hepatic failure when he was undergoing rituximab maintenance therapy. Patients with resolved
HBV, rituximab-based regimen induces reactivation of hepatitis B more frequent than other
chemotherapy regimens, and those patients have a higher rate of mortality compared to HBsAgpositive
cohorts in the mean while. Monitoring serum ALT level monthly with HBV serology
check-up mainly HBsAg (and/or serum HBV DNA) every 3 months in those patients undergoing
rituximab-based chemotherapy are highly recommended.
Keywords: Hbv Reactivation; Resolved Hbv; Rituximab; Lymphoma
Introduction
The chimeric monoclonal anti-CD20 antibody rituximab has been used to treat non-Hodgkin lymphoma (NHL) [1]. Recently, rituximab induced hepatitis B virus (HBV) reactivation in both HBsAg-positive and HBsAg-negative patients were reported [2]. Among these studies, most rituximab therapy was concomitant with chemotherapy including steroid. Whether rituximab maintenance monotherapy could induce HBV reactivation and resulting in serious complications is unknown. Here we report one patient who has negative hepatitis B surface antigen (HBsAgnegative) and positive antibodies to hepatitis B surface antigen (anti-HBs-positive) prior to rituximab-containing chemotherapies develops HBV reactivation followed by fulminant hepatic failure when he was undergoing rituximab maintenance therapy.
Case Presentation
This 57-year-old man presented with progressive enlargement of bilateral neck lymph nodes
and B-cell chronic lymphocytic leukemia with small lymphocytic lymphoma was diagnosed by right
neck lymph node biopsy. He received monthly R-CVP (rituximab, cyclophosphamide, vincristine,
and prednisolone) for three courses followed by five courses monthly R-CHOP (rituximab,
cyclophosphamide, doxorubicin, vincristine, and prednisolone) treatment between September 2009
and February 2010. Serum HBsAg was negative and anti-HBs antibody was positive throughout this
period.
Rituximab monotherapy was administered every twelve weeks as maintenance therapy from
May 2010, and he developed acute jaundice (serum total bilirubin level = 8.1 mg/dL, normal upper limit 1.2 mg/dL) and elevated aminotransferase levels (AST/ALT =
1330/1590 U/L, normal upper limit < 38/50 U/L) seventeen months
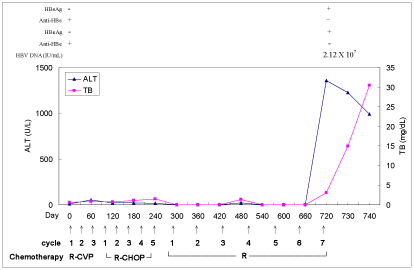
later. HBsAg & HBeAg became positive and serum HBV DNA was
greater than 2.12 X 107 IU/mL (above detection upper limit, realtime
PCR, Taqman method, Roche Amplicor, US) in the meantime
(Figure 1). Anti-HBV agents with entecavir 0.5 mg/day plus
telbivudine 600 mg/day were administered immediately upon HBsAg
positivity reporting; however, the liver enzymes increased gradually
as well as jaundice and coagulopathy in the follow-up studies and he
died of fulminant hepatic failure 20 days after commencing anti-HBV
therapy.
Figure 1
Figure 1
Serum alanine aminotransferase (ALT) and total bilirubin (TB) levels during and after rituximab-based chemotherapy. R-CVP = rituximab,
cyclophosphamide, vincristine, and prednisolone; R-CHOP = rituximab, cyclophosphamide, doxorubicine, vincristine, and prednisolone; R= rituximab.
Discussion
High prevalence of HBV reactivation and mortality rate of
rituximab-based chemotherapy had been reported [3], including
patients with positive hepatitis B surface antigen (HBsAg) and positive
antibodies to hepatitis B surface antigen (anti-HBs). Rituximab-based
chemotherapy (R-CVP and R-CHOP) had been reported to increase
the rate of HBV reactivation in Non-Hodgkin lymphoma patients
with prior resolved HBV status (HBsAg negative and antibody to
hepatitis B core antigen [anti-HBc] positive ± antibody to hepatitis B
surface antigen [anti-HBs] positive) [4].
The rate of HBV reactivation in resolved HBV patients
would increase when anti-HBs titre <100 IU/mL or two lines of
chemotherapies were administered [5,6]. Mutation in pre-core/basal
core promoter and genotype non-A (especially genotype B, C, D)
had been reported to be associated with HBV reactivation [7,8], and
rituximab plus steroid-containing regimen prone to induce more
reactivation of HBV than other chemotherapy regimens [9].
Current HBV treatment guidelines recommend routine HBV
serology test for high risk of HBV infection patients before the
initiation of chemotherapy, and prophylactic anti-HBV agent should
be administered to HBV carriers prior to starting chemotherapy and
maintained for at least 3-6 months after cessation of chemotherapy
[10].
HBsAg-negative patients (including occult and resolved HBV
carriers) were relatively low risk for HBV reactivation when receiving
conventional chemotherapy. Lok et al. [10] reported that HBV
reactivation developed in only 2.7% (2 out of 72) of HBsAg-negative
patients [11]. But Pei et al. [2] demonstrated a 22.1% (15 out of 95) HBV reactivation in HBsAg-negative patients who undergoing rituximab-based therapies.
The clinical course and prognosis of HBV reactivation were worse
than those of acute hepatitis; 27% HBV reactivation group developed
fulminant hepatitis, compared with 7% in the acute hepatitis B group
[12] and in patients with fulminant hepatitis, the HBV reactivation
group had a higher rate of mortality than the acute hepatitis B group
(100% vs. 44%) [12].
According to recent AASLD practice guidelines, HBsAg and
[IgG] anti-HBc test should be performed in patients who have high
risk of HBV infection. Prophylactic antiviral therapy should be
administered to hepatitis B carriers (regardless of baseline serum
HBV DNA level) at the onset of cancer chemotherapy or a finite
course of immunosuppressive therapy, and maintained for 6 months
after cessation of chemo- or immunosuppressive therapies [10]. But
there is no enough information regarding routine prophylaxis for
patients who are HBsAg-negative but anti-HBc or anti-HBs-positive
and in those with isolated anti-HBc-positive.
For HBsAg-negative patients, HBV reactivation happened not
only in Non-Hodgkin lymphoma treatment but also in other cancers
and autoimmune disease treatments. Mastsumoto et al. [13] reported
that reactivation of HBV in patients with resolved HBV after receiving
adalimumab treatment for rheumatoid arthritis.
No standard management to prevent HBV reactivation has been
established for HBsAg-negative patients seropositive for anti-HBc
and/or anti-HBs. According to Japanese guidelines, it is recommended
that serum HBV DNA should be monitored monthly in anti-HBc or
anti-HBs positive patients during and after chemotherapy for at least 1 year [14]. Because virus replication occurs one to two months before
elevation of serum transaminases, prompt antiviral therapy without
delay is suggested once HBV-DNA becomes detectable during follow
up period.
In summary, patients with resolved HBV, rituximab plus steroidcontaining
regimen induces reactivation of hepatitis B more frequent
than other chemotherapy regimens, and those patients have a higher
rate of mortality compared to other cohorts in the meanwhile.
Monitoring serum ALT level monthly with HBV serology check-up
mainly HBsAg (and/or serum HBV DNA) every 3 months in resolved
HBV patients undergoing rituximab-based chemotherapy are highly
recommended.
References
- Czuczman MS, Grillo-Lopez AJ, White CA, Saleh M, Gordon L, LoBuglio AF, et al. Treatment of patients with low-grade B-cell lymphoma with the combination of chimeric anti-CD20 monoclonal antibody and CHOP chemotherapy. J Clin Oncol. 1999; 17: 268-276.
- Pei SN, Chen CH, Lee CM, Wang MC, Ma MC, Hu TH, et al. Reactivation of hepatitis B virus following rituximab-based regimens: a serious complication in both HBsAg-positive and HBsAg-negative patients. Ann Hematol. 2010; 89: 255-262.
- Aksoy S, Harputluoglu H, Kilickap S, Dede DS, Dizdar O, Altundog K, et al. Rituximab-related viral infections in lympnoma patients. Leuk Lymphoma. 2007; 48: 1307-1312.
- Yeo W, Chan TC, Leung NW, Lam WY, Mo FK, Chu MT, et al. Hepatitis B virus reactivation un lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J clin Onco. 2009; 27: 605-611.
- Savas N, Colak T, Yilmaz U, Emiroglu R, Haberal M. Hepatitis B virus reactivation after renal transplantation: report of two cases. Transpl Int. 2007; 20: 301-304.
- Kumagai K, Takagi T, Nakamura S, Sawada U, Kura Y, Kodama F, et al. Hepatitis B virus carriers in the treatment of malignant lymphoma: an epidemiological study in Japan. Ann Oncol. 1997; 8: 107-109.
- Borentain P, Colson P, Coso D, Bories E, Charbonnier A, Stoppa AM, et al. Clinical and virological factors associated with hepatitis B virus reactivation in HBsAg-negative and anti-HBc antibodies-positive patients undergoing chemotherapy and/or autologous stem cell transplantation for cancer. J Viral Hepat. 2010; 17: 807-815.
- Umemura T, Tanaka E, Kiyosawa K, Kumada H. Mortality secondary to fulminant hepatic failure in patients with prior resolution of hepatitis B virus infection in Japan. Clin Infect Dis. 2008; 47: 52-56.
- Hui CK, Cheung WW, Zhang HY, Au WY, Yueng YH, Leung AY, et al. Kinetics and risk of de novo hepatitis B infection in HbsAg-negative patients undergoing cytoxic chemotherapy. Gastroenterology. 2006; 131: 59-68.
- Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009; 50: 661-662.
- Lok AS, Liang RH, Chiu EK, Wong KL, Chan TK, Todd D. Reactivation of hepatitis B virus replication in patients receiving cytotoxic therapy. Report of a prospective study. Gastroenterology. 1991; 100: 182–188.
- Umemura Y, Kiyosawa K. Fatal HBV reactivation in subject with anti-HBs and anti-HBc. Intern Med. 2006; 45: 747-748.
- Mastsumoto T, Marusawa H, dogaki M, Suginoshita Y, Inokuma T. Adalimumab-unduced lethal hepatitis B virus reactivation in an HBsAgnegative patient with clinically resolved hepatitis B virus infection. Liver Int. 2010; 30: 1241-1242.
- Tsubouchi H, Kumada H, Kiyosawa K, Mochida S, Sakaida I, Tanaka E, et al. Prevention of immunosuppressive therapy or chemotherapy-induced reactivation of hepatitis B virus infection: joint report of the Intractable Liver Diseases Study Group of Japan and the Japanese Study Group of the Standard Antiviral Therapy for Viral Hepatitis. Kanzo. 2009; 50: 38-42.