Case Report
Unusual Complication after Paraesophageal Hernia Repair
Tabola R1*, Lewandowski A1, Augoff K1, Zarebski P1, Grabowski K1, Rzechonek A2 and Oleszkiewicz P3
1Department of Gastrointestinal and General Surgery, Medical University, Poland
2Department of Thoracic Surgery, Medical University, Poland
3Department of General and Pediatric Radiology, Medical University, Poland
*Corresponding author: RenataTabola , Department of Gastrointestinal and General Surgery, Medical University, 50-369 Wroclaw, Curie-Sklodowskiej 66, Poland
Published: 02 Sep, 2016
Cite this article as: Tabola R, Lewandowski A, Augoff K,
Zarebski P, Grabowski K, Rzechonek
A, et al. Unusual Complication after
Paraesophageal Hernia Repair. Ann
Clin Case Rep. 2016; 1: 1120.
Abstract
We describe a 66-year old woman with a paraesophageal herniation who was readmitted to our department after previous scheduled repair and an uneventful postoperative course. She had soluble contrast examination and no leak was found from the oesophagus nor was recurrent herniation diagnosed. On computed tomography the abscess was confirmed and reoccurrence of hiatal herniation was excluded. She recovered after the left thoracotomy and evacuation of the purulent hematoma. Mediastinal hematoma is a rare complication that may occur after tearing the arteries in the gastroesophageal junction area, especially if the herniation is approached from the abdominal cavity.
Keywords: Paraesophageal hernia; Complication; Hematoma
Introduction
Hiatal hernia is characterized as protrusion of the stomach into the thoracic cavity through a widening of the right crus of the diaphragm. Large hernias are accidentally diagnosed on plain chest radiographs. There are four types of oesophageal hiatal hernias: sliding (type I), paraesophageal (type II), combined (type III) which is characterised by elements of type I and type II hernias including combination of typical symptoms for both, and giant paraesophageal hiatal hernia (type IV) [1]. Each type may present with different symptoms and specific complications. Sliding hernia accounts for more than 85% of all hernias and is associated with sliding the cardia upward into the thoracic cavity. It results in opening the acute angle between the oesophagus and stomach and promotes reflux, the most essential feature for type I hernias. Type II hernias (paraesophageal) occur in 3.5- 5% of all operated hiatal hernias. The gastroesophageal junction remains in the normal position as an important distinction from other hernias [1-3]. This type of herniation is also characterised by existence of a peritoneal sac, which in some people fails to regress during development and remains bulging into the posterior mediastinum directly, anterior to the oesophagus. This sac may remain empty for the entire life of the patient or the anterior wall of the stomach may protrude into it and compress even the part or the oesophagus, or both [1,4]. These mechanisms explain symptoms. If the lower oesophageal sphincter functions normally, reflux symptoms are rare. A definition of giant paraesophageal hernia is under discussion, but any hernia through the oesophageal hiatus that includes more than one third of the stomach might be considered type IV [1].
Case Presentation
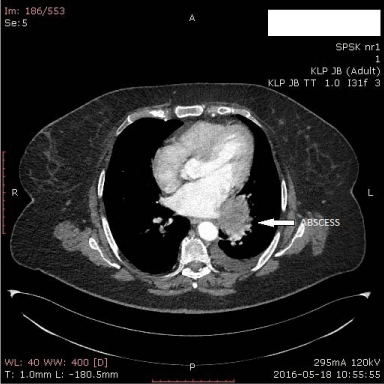
A 66 year old woman was admitted to our department for elective surgery due to symptomatic giant paraesophageal herniation. The herniation was diagnosed on the plain radiograph of the chest and confirmed on gastroscopy and contrast barium examination of the upper gastrointestinal tract. There was no evidence of esophagitis. In the barium swallow study the herniation diameters were 6 x 10 cm in the Trendelenburg’s position. Her complaints were typical for paraesophageal herniation: fullness and vomiting after meals, occasional dysphagia, palpitations, shortness of breath, pain, intolerance of physical activity. Blood tests revealed mild anaemia with Hgb level 11,4g/dL (normal values between12 and 16g/dL). No ischemia was noted on electrocardiogram. She underwent open reposition of the half of the stomach into the abdominal cavity with excision of the sac from abdominal approach. Crura of the diaphragm were approximated and sutured with insoluble suture posteriorly to the oesophagus. Anterior fundoplication was constructed. The postoperative course was uneventful apart from mild dysphagia after solid food. She was dismissed from the hospital on postoperative day 4. She came back to the department on postoperative day 21, complaining for nausea, weakness and sporadic vomiting after meals, she noticed periodic fever. On plain x-ray of the chest she was found to have a cyst behind the heart with fluid level and gas bubble that measured 6,7 x 8,7 cm. She had soluble contrast examination and no leak was found from the oesophagus nor was recurrent herniation diagnosed. On computed tomography the abscess was confirmed and reoccurrence of hiatal herniation was excluded (Figure 1 and 2). Blood tests revealed leukocytosis up to 15,44 x 103 (normal values between 4 and 10x103 μL) and CRP level 13mg/ml (normal range 0.2-5 mg/ml). She was given broad spectrum intravenous antibiotics and scheduled for left thoracotomy for purulent hematoma. The diagnosis was confirmed during the operation. The abscess with a thick rim adjacent to the side wall of the oesophagus, the basis of the left lung and the diaphragm was emptied out of dense pus and blood clots. Its walls were partially cut out. Because dissecting from the oesophagus and the lung parenchyma threatened to damage them for tight adhesions. Two tubes were placed to the pleura and the abscess bed. The patient continued wide spectrum antibiotics. There were no further complications, laboratory parameters of inflammation normalized. Her symptoms completely resolved apart from mild dysphagia for solid food that deteriorated over a period of 2 months since the first surgery.
Discussion
Paraesophageal hernia may present asymptomatically suggesting
intermittent spontaneous reduction [2]. Recently attitude towards
asymptomatic paraesophageal hernias, especially in elder lies has
been modified from immediate surgical repair after the diagnosis to
observation in selected cases [5]. However some authors claim that so
called asymptomatic patients, particularly older ones tend to neglect symptoms that accompany them over a long period of time [6]. The
repair of paraesophageal hernia is more complex than repair of sliding
hernia and not free from complications. Postoperative complication
rate reaches 25% in open approaches [1].
Readmission to hospital after paraesophageal hernia repair
according to Poupore et al. [7] accounts for more than 6% with
median of 11 days to readmission.
The Mediastinal hematoma is a rare complication after hiatal
hernia repair. In our search of the literature we did not find such a
case. Blood supply of the lower portion of the thoracic oesophagus
originates from branches branching off of the left gastric artery,
bronchial artery or directly from the aorta, they might be as small as
1 mm or less in the diameter, thus bleeding from them usually stops
spontaneously [8]. The risk of hematoma emerges during abdominal
approach when the oesophagus or stomach is pulled to reduce the
hernia’s sac or during constructing fundoplication. In 1967 Skinner
et al. [9] describe a variable connecting artery between the left gastric
and left phrenic arteries. The operation in the hiatal area on the lower
esophagus and the gastroesophageal junction may result in tearing
this small artery and cause bleeding that is easily overseen from the
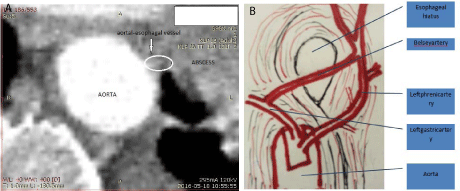
abdominal approach. Figure 3a shows the artery branching off of the
aorta and disappearing in the hematoma, while Figure 3b illustrates
the vessel connecting left phrenic and left gastric arteries. The sac
itself may also poses its own blood supply following the theory that in
some patients with paraesophageal herniation the sac fails to regress
during the development [1]. The sac is dissected bluntly from the
mediastinum and injury to the small vessels during the procedure
remains a possibility, especially there is no complete visual control of
the operative field. Formation of a hematoma around the oesophagus
can be control with a tube placed into the mediastinum through the
hiatal opening for the oesophagus if it is noticed during the surgery. If
the hematoma occurs and is diagnosed early in postoperative period,
its evacuation is possible by thoracostomy. Even thoracotomy is save,
allows for preservation of the reconstructed hiatus and fundoplication,
and removing advanced fibrotic-purulent inflammatory lesions
under visual inspection.
However the diagnosis of such a complication might be difficult.
In differential diagnosis abscess caused by a leak through the damaged
wall of the oesophagus should be considered. Recurrent herniation
and migration of an intact fundoplication must also be excluded. The
diagnosis of recurrence is made by contrast swallow, in some cases
soluble to avoid contamination of the mediastinum if leak is strongly
suspected. Computed tomography is helpful by demonstrating the
position of the stomach, hiatal size and content [10]. We did not worry about the mild dysphagia the patient presented because from
our experience we know it is usually transient for cruroplasty loosens
with time.
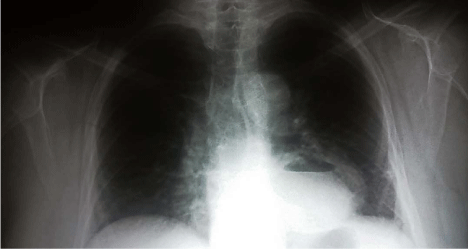
Figure 1
Figure 1
Anterior chest x-ray showing a retrocardiac cyst containing gas
bubble and fluid level which represents the abscess.
Figure 2
Figure 2
Figure 3
(a) Cross-sectional computed tomography showing the artery that
possibly was the cause of hematoma which ended in abscess Arterial phase.
(b) The schematic picture of the so-called Belsey artery connecting the left
gastric and the left phrenic arteries. View from the thoracic cavity.
References
- Dean C, Etienne D, Carpentier B, Gielecki J, Tubbs RS, Loukas M. Hiatal hernias. Surg Radiol Anat. 2012; 34: 291-299.
- Schieman C, Grondin SC. Paraesophageal hernia: clinical presentation, evaluation, and management controversies. Thorac Surg Clin. 2009 ; 19: 473-484.
- Maziak DE, Todd TR, Pearson FG. Massive hiatus hernia: evaluation and surgical management. J Thorac Cardiovasc Surg. 1998; 115: 53-60.
- Matar A, Mroue J, Camporesi E, Mangar D, Albrink M. Large Hiatal Hernia Compressing the Heart. Am J Cardiol. 2016; 117: 483-484.
- Luketich JD, Raja S, Fernando HC, Campbell W, Christie NA, Buenaventura PO, et al. Laroscopic repair of giant paraesophageal hernia: 100 consecutive cases. Ann Surg. 2000; 232: 608-618.
- Altorki NK, Yankelevitz D, Skinner DB. Massive hiatal hernias: the anatomic basis of repair. J Thorac Cardiovasc Surg. 1998; 115: 828-835.
- Poupore AK, Stem M, Molena D, Lidor AO. Incidence, reasons, and risk factors for readmission after surgery for benign distal esophageal disease. Surgery. 2016; 160: 599-606.
- Liebermann-Meffert DM, Luescher U, Neff U, Rüedi TP, Allgöwer M. Esophagectomy without thoracotomy: is there a risk of intramediastinal bleeding?. A study on blood supply of the esophagus. Ann Surg. 1987; 206: 184-192.
- Skinner DB, Belsey RHR. Surgical management of esophageal reflux and hiatus hernia- long term results with 1,030 patients. J Thorac Cardiovasc Surg. 1967; 10: 33-54.
- Chang CG, Thackeray L. Laparoscopic Hiatal Hernia Repair in 221 Patients: Outcomes and Experience. JSLS. 2016; 20.