Case Report
Endoscopic Retrograde Cholangiopancreatography-Related Air Embolism in a Patient with an Unknown Biliary –Venous Fistula and Patent Foramen Ovale: A Case Report
Gómez C*, CuyàsB, Bazaga S, Iborra G and Murzi M>
Department of Gastroenterology and Hepatology, Hospital de la Santa Creu i Sant Pau, Spain
*Corresponding author: Cristina Gómez Oliva, Department of Gastroenterology and Hepatology, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain
Published: 02 Nov, 2016
Cite this article as: Gómez C, CuyàsB, Bazaga S, Iborra
G, Murzi M. Endoscopic Retrograde
Cholangiopancreatography-Related Air
Embolism in a Patient with an Unknown
Biliary –Venous Fistula and Patent
Foramen Ovale: A Case Report. Ann
Clin Case Rep. 2016; 1: 1118.
Abstract
Air embolism is a rare and potentially fatal adverse event associated with endoscopy procedures. We report a case of an endoscopic cholangiopancreatography (ERCP)-related air embolism in a young patient. The diagnosis studies revealed a biliary-venous functional fistula and patent foramen ovale.
Keywords: Endoscopic cholangiopancreatography (ERCP)
Introduction
Air embolism subsequent to an invasive procedure is a rare adverse event which can cause fatal cardiopulmonary compromise. It is described after endoscopy procedures, being ERCP the most frequently related one. A list of 26 reported cases of ERCP-related air embolization are summarized in a recent systematic review [1].
Case Presentation
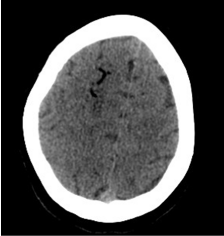
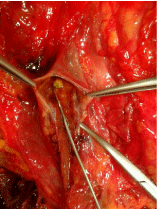
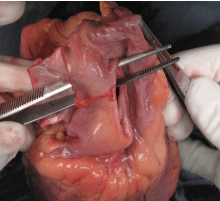
A 32-year-old woman with no relevant medical history was diagnosed by clinical features and magnetic resonance cholangiopancreatography of cholangitis secondary to coledocholithiasis. Therefore, elective ERCP for stone extraction was performed using air insufflation. The patient was deeply sedated with propofol, which was administered by the endoscopy nurse and supervised by the endoscopist, according to guidelines of the American Society of Anesthesiologists (ASA) and European Society of Gastrointestinal Endoscopy [2,3]. Patient monitoring consisted of noninvasive blood pressure, pulse oximetry and clinical assessment. She received oxygen supplementation via an oxygen cannula. After cannulating the common bile duct, a biliary sphincterotomy was done to facilitate the passage of the stone through the distal bile duct. During the procedure, the patient developed livedo reticularis on her face, right brachial area and upper-right abdominal quadrant, respiratory insufficiency (with oxygen saturation below 78%), livedo bradycardia (20 bpm) and hypotension. Both endoscopy procedure and sedation were stopped. However, the patient didn’t wake up, requiring epinephrine, atropine and orotracheal intubation. She developed decerebrate posture, eye deviation and autonomic features. She was finally taken to the intensive care unit (ICU) to recover. Electrocardiogram and emergency transthoracic echocardiogram didn’t show any recognizable abnormality. Brain CT scan and MR imaging showed acute ischemia of the right hemisphere and cerebellum and multiple hypodense/hypointense lesions in the sub cortical white matter, interpreted as being air emboli (Figure 1). Abdominal CT showed a biliary-venous functional fistula. Transesophageal echocardiography failed to detect a patent foramen ovale (PFO). Hyperbaric oxygenation therapy was suggested but finally ruled out due to the impossibility of entering an intubated patient in a hyperbaric chamber.The patient died during the first 24h after the procedure. The autopsy revealed a preserved bile duct (Figure 2) and the existence of a PFO (Figure 3).
Discussion
Different mechanisms for air embolism in ERCP have been proposed, all of them imply a direct communication between a source of air and the circulation. These
include: intramural dissection by the air blown into the portal vein,
transection of duodenal veins during sphincterotomy, biliary-venous
fistulas and shunts, portacaval collaterals, air flow directly into the
hepatic veins or inferior vena cava, retrograde flow into cerebral veins
via superior vena cava, inability of the pulmonary circulation to filter
out gas emboli [1,4-6].
Risk factors for the above mentioned mechanisms are previous
interventions of the bile duct system, post-surgical gastrointestinal
fistula, blunt or penetrating trauma to the liver, transhepatic
portosystemic shunt. Other inflammatory conditions may also
increase the risk of air embolism, such as pylephlebitis, hepatic
abscesses, inflammatory bowel diseases or mesenteric ischemia [4-9].
Air entering the systemic venous circulation causes a major
strain on the right ventricle, which can lead to cardiovascular failure.
Moreover venous embolism can progress into systemic air embolism
via intracardiac or intrapulmonary right to left shunts, retrograde
flow to the cerebral veins through the superior vena cava or by
passage into the left atrium trough the pulmonary veins. The most
common cause of systemic shunt is the existence of a patent foramen
oval, which may be present in 30% of healthy individuals [10].
Once a systemic air embolism occurs a variety of cardiovascular,
pulmonary and neurological symptoms can appear. Cardiovascular findings include arrhythmia, hypotension, myocardial ischemia,
right heart failure and cardiac arrest. Pulmonary symptoms include
acute dyspnea, tachypnea, breathlessness, rales, wheezing, hypoxia,
cyanosis and respiratory failure. Finally, neurological symptoms
include eye deviation, dilated pupils, unconsciousness, hemiparesis,
cerebral edema and coma [11].
Due to its low incidence, diagnosis of air embolism may be
difficult. It is necessary to maintain a high index of suspicion and to
exclude other severe pathologies.
When air embolism is suspected initial measures should be
started while the definitive diagnosis is established. Such manoeuvres
include: stopping the procedure if possible, administer high flux
oxygen, initiate fluid therapy and place the patient in trendelenburg
and left lateral position [12].
Once those are completed directed diagnosis studies should be
performed to assess the final diagnosis. Bedside echocardiogram may
allow the visualization of air within the right heart, brain and thorax
CT scan can confirm the diagnosis [1].
Specific treatment includes air aspiration via a central venous
catheter; hyperbaric oxygenation therapy has also been suggested as
a possible treatment, considering its lack of availability in most of the
centres.
In the case of cardiopulmonary arrest, cardiopulmonary
resuscitation should be initiated in other to maintain the cardiac
output.
The use of CO2 insufflation has been widely compared to air
insufflation. In a meta-analysis comparing CO2 and air insufflation
during ERCP, CO2 insufflation was associated with significantly
lower pain perception during the first 6 hours post procedure.
To prevent air embolism, the American Society for
Gastrointestinal Endoscopy (ASGE) has recently recommended
using CO2 instead of air insufflation. Although air embolism has
been described in procedures using CO2 insufflation, overall, CO2
embolism is reasonably well-tolerated and therefore, preferred
insufflation gas form [13].
Figure 1
Figure 2
Figure 3
Conclusion
In conclusion, establishing an early diagnosis of air embolism would improve the outcome in these cases, reducing mortality. As no specific treatment is described for air embolism detecting and avoiding, risk factors should be a priority. CO2 insufflation during endoscopic procedures is associated with less post procedural pain compared with air insufflation. As recently recommended by ASGE, CO2 insufflation should be offered in patients undergoing endoscopic procedures, especially when they are associated with a higher risk of perforation or gas embolism.
References
- Donepudi, Chavalitdhamrong D, Pu L, Draganov PV. Air embolismcomplicating gastrointestinal endoscopy: A systematicreview. World J Gastrointest Endosc. 2013; 5: 359-65.
- Dumonceau JM, Riphaus A, Schreiber F, Vilmann P, Beilenhoff U, Aparicio JR, et al. Non-anesthesiologist administration of propofol for gastrointestinal endoscopy: European Society of Gastrointestinal Endoscopy, EuropeanSociety of Gastroenterology and Endoscopy Nurses and Associates Guideline. NAAP: ESGE-ESGENA Guideline 2015. Endoscopy. 2015; 47: 1175–1189.
- American Society of Anesthesiologists. Practiceguidelinesforsedation and analgesia by non-anesthesiologists. Anupdatereportbythe ASA TaskForceonSedation and Analgesia by Non-anesthesiologists. Anesthesiology. 2002; 96: 1004-1017.
- Bisceglia M, Simeone A, Forlano R, Andriulli A, Pilotto A. Fatal systemicvenous air embolismduringendoscopic retrograde cholangiopancreatography. Adv Anat Pathol. 2009; 16: 255-262.
- Thackray NM, Murphy PM, McLean RF, deLacy JL. Venous air embolism accompanied by echocardiographic evidence of transpulmonary air passage. Crit Care Med. 1996; 24: 359-361.
- Hauser G, Milosevic M, Zelić M, Stimac D. Sudden death after endoscopic retrograde cholangiopancreatography (ERCP)-case report and literature review. Medicine (Baltimore). 2014; 93: e235.
- Maccarone G, Shakoor T, Ellis B. Air embolism after percutaneous transhepatic biliary drainage and subsequent endoscopic retrograde cholangiopancreatography (ERCP). Endoscopy. 2011; 43: E399.
- Finsterer J, Stöllberger C, Bastovansky A. Cardiac and cerebral air embolism from endoscopic retrograde cholangio-pancreatography. Eur J Gastroenterol Hepatol. 2010; 22: 1157-1162.
- Green BT, Tendler DA. Cerebral air embolism during upper endoscopy: case report and review. Gastrointest Endosc. 2005; 61: 620-623.
- Lynch JJ, Schuchard GH, Gross CM, Wann LS. Prevalence of right-to-left atrial shunting in a healthy population: detection by Valsalva maneuver contrast echocardiography. Am J Cardiol. 1984; 53: 1478-1480.
- Mirski MA, Lele AV, Fitzsimmons L, Toung TJ. Diagnosis and treatment of vascular air embolism. Anesthesiology. 2007; 106: 164-177.
- Rangappa P, Uhde B, Byard RW, Wurm A, Thomas PD. Fatal cerebral arterial gas embolismafterendoscopic retrograde cholangiopancreatography. Indian J Crit Care Med. 2009; 13: 108-112.
- Simon K Lo, Larissa L Fujii-Lau, Brintha K Enestvedt, Joo Ha Hwang, Vani Konda, Michael A Manfredi, et al. The use of carbondioxide in gastrointestinal endoscopy. Gastrointest Endosc. 2016; 83: 857-865.