Case Report
A Case of Osteomyelitis in Patient with Remaining Assist Device Driveline after Heart Transplantation: A Case Report
Harmel E1*, Barten MJ1, Philipp S2, Rybczynski M1, Reichenspurner H1 and Sill B1
1Department of Cardiac Surgery, University Heart Center Hamburg, Germany
2Department of Cardiology, Elbe Kliniken Stade, Germany
*Corresponding author: Eva Harmel, Department of Cardiac Surgery, University Heart Center Hamburg, Gebäude O70, Martinistr. 52, 20246 Hamburg, Germany
Published: 29 Aug, 2016
Cite this article as: Harmel E, Barten MJ, Philipp S,
Rybczynski M, Reichenspurner H, Sill
B. A Case of Osteomyelitis in Patient
with Remaining Assist Device Driveline
after Heart Transplantation: A Case
Report. Ann Clin Case Rep. 2016; 1:
1108.
Abstract
Introduction: Since the development of ventricular assist devices (VADs), they have been
used as a bridge to heart transplantation in patients with end-stage heart failure. Therefore, an
increasing number of patients receive heart transplantations from this device. As is common
for re-do procedures, numerous tissue adhesions are found and the operative procedure for the
heart transplantation becomes more challenging. The time-consuming approach often leads to an
incomplete removal of driveline and outflow graft material.
Case Presentation: We report about a 49-year old male patient with ischemic cardiomyopathy
who underwent implantation of a left ventricular assist device in 2013 and orthotopic heart
transplantation in 2014. Twelve months after transplantation, the patient was admitted to the
hospital with painful swelling and left-sided chest pain. Laboratory results showed a slight increase
of inflammatory markers. Multiple blood culture sets remained negative. Computer tomography
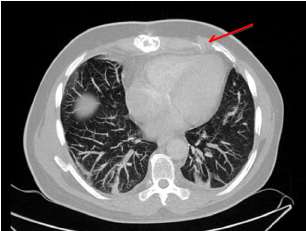
(CT) scans and magnetic resonance imaging (MRI) showed signs of osteomyelitis of the left fourth
rib. After operative rib excision, microbiological testing revealed colonization of osteoid tissue with
C. albicans and S. epidermidis. An antibiotic and antifungal therapy was initiated, followed by wound
treatment with vacuum assisted closure (VAC) therapy. Due to persistent pain despite opioids, the
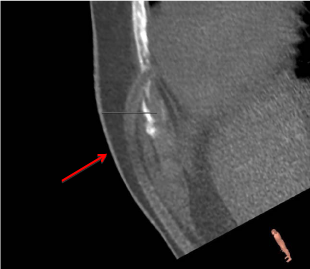
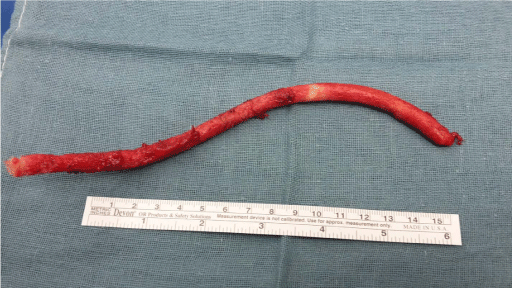
thoracic images were reevaluated. A 20 cm long piece of remaining driveline was identified, located
in the area of left sided 4th to 5th intercostal space. After operative removal of the driveline, a sudden
pain relief and decrease of inflammatory markers was seen.
Conclusion: In long-term support with ventricular assist devices, driveline infections are still one
of the key problems. Whether this also affects remaining driveline after heart transplantation is still
not known. Due to the obligatory immunosuppression, the general risk for infection is increased.
Therefore the complete excision of the ventricular assist device, including the removal of all foreign
material, seems to be the most appropriate approach.
Introduction
Although heart transplantation remains the gold standard in treatment of advanced or endstage heart failure, it is limited by the number of available donor organs. Implantable ventricular assist devices (VADs) have been developed to work as tools for long-term therapy in patients with advanced heart failure, or as bridges to transplant. Their development might be one of the key tools to counter organ scarcity. Nevertheless, it is known that despite the technological improvements, VAD therapy is still related to a large number of side effects. Complications such as bleeding, driveline infection, pump thrombosis, and stroke are causing an increasing number of patients receiving heart transplantation from the device (VAD) [1]. The 2015 annual INTERMACS report revealed that approximately one third of patients with a VAD received heart transplantation within twelve months [2]. As common for re-do procedures, numerous tissue adhesions are found and the operative procedure of transplantation becomes more challenging. Due to oral anticoagulation during VAD therapy, surgeons have to deal with increased bleeding and prolonged hemostasis. Despite the fact that short ischemia and cardiopulmonary bypass time remain key goals to improve outcome, operative strategies for how to deal with the remaining driveline after VAD explantation are necessary.
Case Presentation
We report about a 49-year old male Caucasian patient with ischemic cardiomyopathy. The risk factors for cardiovascular disease included arterial hypertension, hyperlipidemia, and smoking (30 pack-years) but no diabetes. The patient’s past medical history includes two ST-elevation myocardial infarctions (STEMI) in 2009 and 2011 with percutaneous coronary intervention. This was followed by an implantable cardioverter defibrillator (ICD) and cardiac bypass surgery (LIMA to LAD and RIM) in 2012. Due to progressive left heart failure, the patient underwent implantation of a continuous flow left ventricular assist device (HeartWare) in 2013. After thromboembolic complication (right cerebral infarction) during device support, the patient was listed for heart transplantation and got transplanted in 2014. Twelve months after transplantation the patient was admitted to the hospital with painful swelling and left-sided chest pain. Apart from mild left pectoral swelling and tenderness on palpation, local findings did not reveal further signs of inflammation. Laboratory results showed only a slight increase of inflammatory markers, such as C-reactive protein or white blood cell count. Multiple blood culture sets remained negative. Because of persistent and progressive pain, further imaging was used. Computer tomography (CT) and magnetic resonance imaging (MRI) showed signs of osteomyelitis in the ventral parts of the left fourth rib, in close proximity to the right ventricle. Therefore, operative excision of the ventral parts of the fourth rib was performed and osteoid material was sent for microbiological testing. A colonization of osteoid tissue with C. albicans as well as S. epidermidis was revealed. The antifungal sensitivity profile showed a Minimum Inhibitory Concentration (MIC) of 0.032 μg/ml for amphotericin and a MIC of 0.25 μg/ml for fluconazole. The antibiotic sensitivity profile for S. epidermidis proved sensitivity to second and fourth generation fluoroquinolones (ciprofloxacin and moxifloxacin), narrow-spectrum beta-lactam antibiotics (flucloxacillin) as well as reserve antibiotics such as linezolid, teicoplanin, vancomycin, or rifampicin. According to the anti-biogram, an anti-infective therapy with ciprofloxacin and flucloxacillin was initiated, as well as an antifungal therapy with fluconazole. The wound was treated with vacuum assisted closure (VAC) therapy. Wound debridement and changes of dressing were performed every 3-4 days. After three negative microbiological results, VAC treatment was terminated and secondary wound closure was performed. Due to persistent pain despite chronic opioid pain medicine, the thoracic images (CT and MRI scans) were re-evaluated. An approximately 20 cm long piece of remaining driveline was identified, located in the area of left-sided 4th to 5th intercostal space. Indication for operative removal was set. Operative excision of the extra thoracic located piece of driveline was carried out. The perioperative and early postoperative follow-up did not show any abnormalities. Local wound findings were absent for signs of inflammation. Laboratory results showed a sudden decrease of white blood cell count and C-reactive protein. The patient was discharged home. The four-week follow-up in the outpatient clinic showed pain relief and a well-healing wound. Local findings showed a reduction of seroma.
Figure 1
Figure 2
Figure 2
Reconstruction model (3 mension) of the remaining piece of
driveline in location to the leftsided 4th to 5th intercostal space.
Figure 3
Discussion
Due to an increasing number of patients with ventricular assist devices, orthotopic heart transplantation is more and more performed as a re-do procedure, sometimes even after several previous cardiothoracic operations. In this case, previous to the heart transplantation, the patient already had an implantable cardioverter defibrillator (ICD), received bypass surgery, and received implantation of a left ventricular assist device. Repeated and previous cardiothoracic operations cause heart transplantation to become more technically challenging and the approach via sternotomy often takes a lot of time due to massive adhesiolysis. The time-consuming approach frequently leads to an incomplete removal of driveline and outflow graft material. It is known that in patients with long-term VAD support, driveline infections are still one of the key problems. Zierer et al. [3] described a rate of late-onset driveline infections occurring in almost all patients with support duration over one year. Despite the fact that infection control is improving, the seventh INTERMACS report still revealed an assist device-related infection in over thirty percent of patients during the first three years after implantation [2]. Whether this also affects remaining driveline after heart transplantation is still not known. But due to obligatory immunosuppression, the general risk for infection in patients after heart transplantation is increased. The presence of chronic infection seems to be a significant risk factor for reoperation after assist device removal [4]. In the described case the immunosuppression regime was set with tacrolimus (Prograf; target blood concentration: 6-8 ng/ml), mycophenolate sodium (Myfortic; dosage: 360 mg 1-1-1) and prednisolone (dosage: 7,5 mg 1-0-0). Apart from the immunosuppressive therapy listed above, the medical history was negative for other risk factors of infection. In detail, this middle-aged patient didn’t suffer from obesity nor was he underweight. There was no history of diabetes, no known underlying disease affecting the immune system, and no history of cancer. Therefore one must consider that a chronic infection, caused by the remaining driveline material, led to osteomyelitis in this patient. Due to the risk of infectious complications, the complete VAD excision including the removal of all foreign material represents the most appropriate approach [4]. In the described case, remaining driveline caused persistent pain and several re-admissions to hospital. After heart transplantation in 2014, the patient spent over fifty percent of his time in the hospital, accompanied by social isolation, reduced quality of life, and inability to work.
References
- Frazier OH, Baldwin AC, Demirozu ZT, Segura AM, Hernandez R, Taegtmeyer H, et al. Ventricular reconditioning and pump explantation in patients supported by continous-flow left ventricular assist devices. J Heart Lung Transplant. 2015: 766-772.
- Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015; 34: 1495-1504.
- Zierer A, Melby SJ, Voeller RK, Guthrie TJ, Ewald GA, Shelton K, et al. Late-onset driveline infections: the Achilles' heel of prolonged left ventricular assist device support. Ann Thorac Surg. 2007; 84: 515-520.
- Baldwin AC, Sandoval E, Letsou GV, Mallidi HR, Cohn WE, Frazier OH. Surgical approach to continuous-flow left ventricular assist device explantation: A comparison of outcomes. J Thorac Cardiovasc Surg. 2016; 151: 192-198.