Case Report
Pneumo-Enzymatic Vitreolysis for Resistant Vitreomacular Traction
Jorge G. Arroyo1*, Gina Yu1, Rachel Tandias1 and Sushant Wagley2
1Department of Ophthalmology, Harvard Medical School, USA
2Hurley Medical Center, USA
*Corresponding author: Jorge G. Arroyo, Department of, Ophthalmology/CC5, 330 Brookline Avenue, Boston, MA 02215-5400, USA
Published: 25 Aug, 2016
Cite this article as: Arroyo JG, Yu G, Tandias R, Wagley
S. Pneumo-Enzymatic Vitreolysis for
Resistant Vitreomacular Traction. Ann
Clin Case Rep. 2016; 1: 1104.
Abstract
Aim: Case report describing a patient with resistant vitreomacular traction (VMT) treated with an
intravitreal injection of a potentially safer, lower dose of ocriplasmin and with a residual intravitreal
gas bubble from pneumatic vitreolysis.
Case Report: An 88-year-old man with symptomatic vitreomacular traction (VMT) was observed for
4 weeks without spontaneous release. Pneumatic vitreolysis (PV, intravitreal injection of expansile
gas with intermittent face-down positioning [FDP]) was performed. Four weeks later, a 30% bubble
was still present in the vitreous, vision remained 20/70, and he had improved but persistent VMT.
One-half of the recommended therapeutic dose of ocriplasmin was injected intravitreally and the
patient continued FDP. At one-week follow-up, the patient had improved vision of 20/40, associated
with complete VMT release. He had no adverse events.
Discussion: Resistant VMT can lead to significant foveal distortion and subsequent vision loss.
Pneumatic vitreolysis appears to successfully relieve VMT in about 80% of cases, but in the 20% of
cases that do not respond, typically invasive, surgical intervention is required. This report shows the
efficacy of combining the mechanical forces of PV with the enzymatic fibrinolytic activity available
in a potentially safer, lower dose of ocriplasmin to treat resistant VMT cases, non-invasively.
Summary: For resistant vitreomacular traction, combining the mechanical force of an intravitreal
injection of an expansile gas with a half-dose of ocriplasmin could provide an efficacious treatment
option, without the risk of the toxic safety profile often associated with ocriplasmin.
Keywords
Ocriplasmin; Posterior vitreous detachment; Traction
Introduction
Vitreomacular traction (VMT) results when abnormally strong vitreomacular adhesions
(VMA) between the vitreous and retina cause tugging of the retina as the vitreous contracts,
resulting in distortion of the neurosensory retina which can lead to vision loss [1]. Though pars
plana vitrectomy with membrane peeling (PPV/MP) is the gold standard treatment for VMT, the
introduction of ocriplasmin (Jetrea®; ThromboGenics Inc, USA, Alcon/Novartis EU) presented a
potentially less invasive option. In the microplasmin for Intravitreal Injection – Traction Release
without Surgical Treatment (MIVI-TRUST) clinical trials, 26.5% of patients with VMT experienced
release compared to 10.1% (p<0.001) of control patients [2]. With subsequent increased use of
ocriplasmin, there have been several reports of unexplainable adverse events like transient vision
loss, outer retinal degeneration, and photopsia [2,3].
Pneumatic vitreolysis (PV, the intravitreal injection of an expansile gas with intermittent face
down positioning) has been found to be relatively safe and effective in releasing VMT in up to 0.84
(95% CI: 0.76-0.92) of patients as analyzed via meta-analysis [4,5]. It is believed to work by inducing
a mechanical posterior vitreous detachment (PVD) from injection of an expansile gas, like C3F8
[5]. Previous studies have compared intravitreal ocriplasmin (IVO) and PV alone, but a combined
approach has not been explored [4].
Although PV appears to be effective in a majority of VMT patients, about 20% of patients fail
to experience VMT release. This case report demonstrates the apparent efficacy of using a halfrecommended
dose of ocriplasmin in patients who do not experience VMT release with PV alone
at 1 month. A lower dose of ocriplasmin, like the one presented, may reduce the complications
associated with the enzymatic activity of ocriplasmin.
Case Presentation
An 88-year old man presented at Beth Israel Deaconess Medical
Center, Boston, with a visually significant VMT in his right eye. He
had noticeably decreased vision for 6 weeks prior to consultation.
In addition to VMT, he had bilateral moderate dry age-related
macular degeneration. His best-corrected visual acuity was 20/70+2
in his right eye. An ocular coherence tomographic (OCT) scan
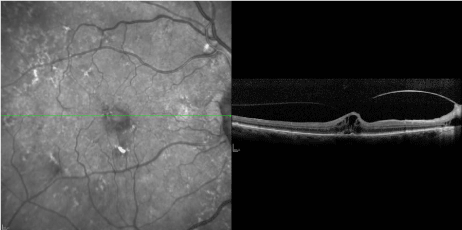
showed VMT without any evidence of a macular hole (Figure 1).
He was observed for four weeks without change in vision or VMT
release. Surgical and non-surgical treatment options were discussed
and he elected to undergo PV, with a 0.3 cc intravitreal injection
of 100% perfluoropropane (C3F8) gas (Alcon ISPAN), done in the
office following standard sterile intravitreal injection techniques,
as described in a previous publication [4]. Briefly, the patient
was given topical anesthetic lidocaine drops, a wire lid speculum
was placed, and 5% and 10% povidone-iodine (Betadine) was
provided as a disinfectant. The intravitreal injection was performed
superotemporally using a 30-gauge needle, 4 mm posterior to
the limbus. The 0.3 cc of 100% C3F8 gas was injected intravitreally
through the pars plana using a 30-gauge needle and a 1 mL syringe.
A paracentesis was also performed to decrease intraocular pressure.
After the procedure, the patient was instructed to remain face down
15 minutes every 30 minutes while awake for 4 weeks.
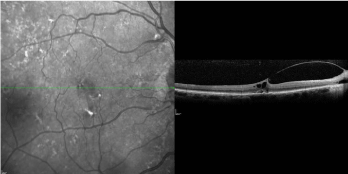
At day 28, the VMT had not resolved, despite good facedown
positioning (Figure 2). The patient then underwent a single
intravitreal injection of 62.5 μg of ocriplasmin (half the recommended
therapeutic dose). The treatment was provided using sterile injection
techniques within the FDA-approved package insert guidelines for
ocriplasmin.
The patient noticed significant visual improvement 1-2 days after
treatment, and at his day 7 post-treatment visits, he was found to
have an improved visual acuity at the 20/40 level with complete VMT
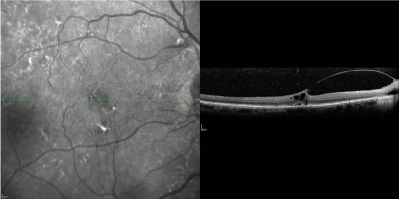
resolution (Figure 3). He did not develop any macular holes, retinal tears or breaks, retinal detachments, or endophthalmitis during his treatments.
Discussion
Patients with symptomatic VMT may benefit from treatment with PPV/MP, ocriplasmin, or PV [2,5]. This case presents a novel combination treatment for resistant VMT that may provide better outcomes than either PV or ocriplasmin injection alone, and potentially with a lower overall complication rate. Complications associated with IVO have included photopsia, dyschromatopsia, and ultrastructural and electroretinographic changes, and the exact cause for such adverse events is still not well understood [3,6,7]. Due to the uncertain safety profile and longer-term after-effects of this still relatively new pharmacologic agent, other treatment options such as PV appear especially promising. In the approximately 20% of patients [4] who do not have VMT release with PV at 1 month and still have a residual gas bubble in the eye, combination treatment with a halfdose IVO may provide an option that combines the mechanical force of PV with the enzymatic fibrinolytic activity from IVO. This combined treatment also reduces the risk of toxicity by using a lower dose of ocriplasmin. Randomized controlled trials of PV followed by low dose IVO are necessary to better investigate this promising combination treatment strategy and associated risks in the future. This case report serves as a starting point for further investigations examining the efficacy of this combined approach.
Figure 1
Figure 1
OCT image taken at patient’s initial consultation. Visual acuity at
time point was 20/70+2.
Figure 2
Figure 2
OCT image taken 28 days after an intravitreal injection of 0.3 mL of
100% C3F8. Visual acuity at time point was 20/70+2.
Figure 3
Figure 3
OCT image taken 7 days after injection of half-dose of ocriplasmin.
Visual acuity at time point improved to 20/40.
References
- Hikichi T, Yoshida A, Trempe CL. Course of vitreomacular traction syndrome. Am J Ophthalmol. 1995; 119: 55-1.
- Stalmans P, Benz MS, Gandorfer A, Kampik A, Girach A, Pakola S, et al. Enzymatic vitreolysis with ocriplasmin for vitreomacular traction and macular holes. N Engl J Med. 2012; 367: 606-615.
- Thanos A, Hernandez-Siman J, Marra KV, Arroyo JG. Reversible vision loss and outer retinal abnormalities after intravitreal ocriplasmin injection. Retin Cases Brief Rep. 2014; 8: 330-332.
- Yu G, Duguay J, Marra KV, Gautam S, Le Guern G, Begum S, et al. Efficacy and Safety of Treatment Options for Vitreomacular Traction: A Case Series and Meta-Analysis. Retina. 2016; 36: 1260-1270.
- Rodrigues IA, Stangos AN, McHugh DA, Jackson TL. Intravitreal injection of expansile perfluoropropane (c(3)f(8)) for the treatment of vitreomacular traction. Am J Ophthalmol. 2013; 155: 270-276.
- Freund KB, Shah SA, Shah VP. Correlation of transient vision loss with outer retinal disruption following intravitreal ocriplasmin. Eye. 2013; 27: 773-774.
- Fahim AT, Khan NW, Johnson MW. Acute panretinal structural and functional abnormalities after intravitreous ocriplasmin injection. JAMA Ophthalmol. 2014; 132: 484-486.