Case Report
A Combined Overdose of Diltiazem and Amlodipine: A Challenging Case
Ali-Ahmed F1*, Demetriou M2 and Berman A3
1Internal Medicine, Beaumont Health, USA
2Wayne State University, USA
3Department of Cardiovascular Medicine, Beaumont Health, USA
*Corresponding author: Fatima A Ali-Ahmed, Department of Internal Medicine, Beaumont Health, 3535 W 13 Mile Rd, Royal Oak, Michigan, 48073, USA
Published: 25 Aug 2016
Cite this article as: Ali-Ahmed F, Demetriou M, Berman A.
A Combined Overdose of Diltiazem and
Amlodipine: A Challenging Case. Ann
Clin Case Rep. 2016; 1: 1099.
Abstract
Calcium channel blocker (CCB) toxicity can be devastating, unpredictable, and complicating. A 65-year-old female with a history of hypertension and atrial fibrillation presented with altered mental status, hypotension, and bradycardia at 34 bpm. She quickly deteriorated, developing profound refractory bradycardia and hypotension; despite aggressive IV fluids, atropine, naloxone, glucagon pushes, and support with dopamine. A transvenous pacemaker (TVP) was placed, but would not capture. Given the lack of response to therapy, her medication bottles were reviewed again, revealing empty bottles of amlodipine 10 mg and diltiazem XL 240 mg. She was then bolused with calcium gluconate and switched to norepinephrine, after which, the TVP started capturing; however, she continued to display refractory hypotension, requiring three pressors. She was then placed on high dose insulin (HDI) drip of 1-8 units/kg/hr, with glucose, and weaned off pressors. The insulin drip was titrated upwards until the hypotension resolved. After 24 hours of HDI, the patient made a full recovery. CCBs, such as dihydropyridines and nondihydropyridines, block L-type calcium channels in the myocardium or vascular smooth muscle, causing depression of the conduction system, inability to pace, and refractory hypotension. Patients may fail initial therapies, requiring IV calcium, HDI, or TVP. Since CCBs are highly protein-bound, dialysis provides no benefit, and glucagon is of questionable usefulness. The therapeutic role of insulin is to optimize the glucose-dependent energy formation required to overcome CCB overdose. Consequently, while it is difficult to recommend one specific strategy, early recognition is imperative for effective management and urgent intervention. Keywords: Amlodipine; Calcium channel blocker; Diltiazem; Overdose; Toxicity
Introduction
Calcium channel blockers (CCBs) have many clinical applications, including therapy of
hypertension, arrhythmia, angina, and various circulatory conditions, due to their inhibition of
calcium influx through L-type calcium channels residing on the myocardium and vascular smooth
muscle [1]. Despite these beneficial uses, the toxicity of CCB overdose (intentional or otherwise)
can be lethal, potentially causing serious life threatening complications such as profound refractory
bradycardia, hypotension, and poison-induced cardiogenic shock or even death [1].
CCBs are divided into two categories, dihydropyridines (e.g., amlodipine) or nondihydropyridines
(e.g., diltiazem) blockers. Dihydropyridine (DHP) CCBs mainly serve as a potent peripheral
vasodilators, with minimal effects on cardiac contractility or conduction; at toxic doses, however,
this receptor selectivity is lost, potentially leading to myocardial depression, bradycardia, and
atrioventricular node blockade [1,2]. Nondihydropyridine (non-DHP) CCBs predominantly act on
the myocardium, affecting cardiac contractility, sinoatrial (SA) node function, and atrioventricular
(AV) nodal conduction [1,2]. Consequently, non-DHP overdose is potentially the most lethal CCB
toxicity.
When DHP or non-DHP CCBs is ingested in large doses, their cardiovascular and vascular
effects can produce profound refractory bradycardia and hypotension; thus, treating patients is
often challenging. Herein, we describe a case of a combined overdose of amlodipine (DHP) and
extended-release diltiazem (a non-DHP CCB) causing a catastrophic poison-induced cardiogenic
shock.
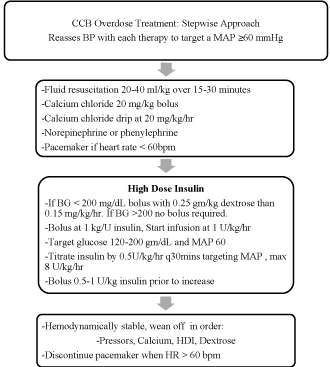
Figure 1
Figure 1
Treatment of CCB overdose base on the recommendations of Children's Hospital of Michigan Regional Poison Control Center, Detroit, MI. Mean arterial
pressure (MAP), Units (U), Blood glucose (BG), High does insulin (HDI).
Case Presentation
A 65-year-old female with a history of hypertension and atrial fibrillation presented with altered mental status and bradycardia. According to a family member, the patient appeared confused, prompting a call to EMS. She was last seen in her normal baseline of health about 6-8 hours ago. While en route, the patient was hypotensive and bradycardic. She received IV fluids and atropine 2 mg, which slightly improved her blood pressure. On presentation, the patient was hypotensive, with a blood pressure of 61/39 mmHg, and bradycardic at 34 bpm. She stated, “I took a few extra pills of my beta blocker”. She was drowsy, but alert and oriented, and requested DNR/ DNI status. The patient quickly deteriorated, developing profound, refractory bradycardia and hypotension, despite aggressive IV fluids, repeat atropine, naloxone, glucagon pushes, and pressor support with dopamine. She was lethargic and unresponsive, and with no family members to be found, her overdose was deemed a suicide attempt, and she was subsequently intubated. A transvenous pacemaker (TVP) was placed but would not capture. Given the patient’s lack of response to therapy, her medication bottles were reviewed again, revealing empty bottles of amlodipine 10 mg and diltiazem XL 240 mg prescribed tablets. It was felt that the combination of these two agents led to depression of the conduction system and severe vasodilatation, causing severe bradycardia, inability to pace, and refractory hypotension. A bolus and infusion of calcium gluconate was administered, and then switched to norepinephrine. Afterward, the TVP started capturing; however she continued to display refractory hypotension, requiring maximum support of norepinephrine at 30 mcg/min, dopamine 15 mcg/kg/min, and epinephrine 5 mcg/min. Following the increased requirement of pressure support, she was bolused with 55 units of insulin (1 unit/kg/), and placed on a high-dose (1-8 unit/kg/hr) insulin drip, with dextrose 10% at 100 cc/hr and dextrose 5% pushes, as needed. The high-dose insulin (HDI) drip was titrated by 0.5 mg/kg/hr every 30 minutes to maintain a mean arterial pressure (MAP) ≥60 mmHg, while simultaneously weaning pressors. In addition, the patient was bolused with 1 unit/kg of insulin prior to any increase in infusion rate. She required a maximum of 7 units/ kg/hr of insulin (385 units). Due to the large amount of dextrose required to maintain the patient’s blood glucose between 120-200 mg/dL, she was switched to dextrose 20% and 37.7 g of dextrose 50% every 30 minutes, as needed, to reduce her risk of third spacing, due to the vasodilatory effect of CCB, thus reducing the risk of pulmonary edema. Within 12 hours of starting the HDI, the patient was weaned off all pressors and the TVP was discontinued. After about 20 hours of high-dose insulin, the patient’s bradycardia and hypotension resolved. Her HDI was weaned down by 1 unit/kg every 30 minutes, requiring HDI for a total of 24 hours. The following day, the patient was extubated. She made a full recovery and was discharged to an inpatient psychiatry unit a few days later.
Discussion
CCB toxicity is one of the most common causes of poisoninduced
cardiogenic shock (PCIS), and is the second-leading cause of
death of any cardiovascular medication [3,4]. In 2014, the American
Association of Poison Control Centers reported 12,007 cases of CCB
toxicity, resulting in 86 major outcomes and 20 deaths [4].
CCBs inhibit myocardial contractility, decrease cardiac
automaticity, and reduce peripheral vascular resistance. Therefore,
the typical hallmark of CCB toxicity is bradycardia, conduction
blocks, and hypotension [1,2]. In CCB toxicity, the myocardium
becomes stressed, switching to carbohydrate metabolism, and
shifting away from normal cardiac utilization and free fatty acid
oxidation [5]. Yet, at the same time, CCBs create a vicious cycle, by
preventing adequate myocardial utilization of carbohydrates during
a stress, which is critical during shock. The proposed mechanism of
this toxicity is inhibition of insulin secretion by pancreatic beta-islet
cells, leading to insulin resistance, combined with depressed cardiac
output, thus reducing delivery of insulin and glucose [5,6].
The primary goals of PCIS interventions are to preserve organ
perfusion and restore hemodynamic stability [7]. Initial treatments
for CCB overdose may include airway maintenance, gastrointestinal
decontamination, and fluids, in hemodynamically stable patients
[8]. However, patients often quickly deteriorate, requiring further
interventions, such as atropine, IV calcium, high-dose insulin, and/or
transvenous pacemaker (TVP) [2,9]. Special attention should be given
to cardiac pacing, as it may not result in capture, due to impaired
conduction and no improvement in blood pressure, as demonstrated
in our patient. Since CCBs are highly protein-bound, dialysis
provides no benefit, and glucagon is of questionable usefulness in
CCB overdose [10].
For patients who fail to respond to initial treatments, highdose
insulin (HDI) may serve as a second line therapy (Figure 1).
Although there are no prospective clinical studies comparing HDI
to conventional treatments, a review of the literature, which includes
animal studies and human case series, supports the use of HDI,
demonstrating superior treatment in terms of safety and survival [11-
13]. The proposed beneficial mechanism of insulin involves improved
smooth muscle contractility via increased efficiency of carbohydrate
uptake. Such uptake counteracts CCB blockade, while also optimizing
the glucose-dependent energy formation required to overcome CCB
overdose, therefore improving cardiac function [14].
CCB overdose (intentional or unintentional) is a life-threatening
condition that requires urgent intervention. As demonstrated in our
case, CCB overdose can be potentially lethal, due to profound refractory
bradycardia, cardiogenic shock, and hypotension. Consequently,
it is difficult to recommend one specific strategy; however, urgent
administration of fluids, calcium, atropine, vasopressors, and insulin
therapy appear to be the mainstay approach [1].
Conclusion
Calcium channel blocker (CCB) overdose is a life-threatening event requiring urgent initiation of management. The American Association of Poison Control Centers has recommended that CCB overdose be strongly considered as intentionally self-harmful, and that known suicidal cases be immediately transported by ambulance to a hospital emergency department [4]. As demonstrated in our case, signs of CCB toxicity include bradycardia, lethargy, and/or refractory hypotension [1,2]. Based on our case report and a review of literature, patients with near fatal CCB overdose, if recognized rapidly, can be effectively managed by rigorous, and urgent interventions such as HDI, particularly in patients who failed traditional therapies.
References
- Burkes, R, Wendorf G. A multifaceted approach to calcium channe blocker overdose: A case report and literature review. Clin Case Rep. 2015; 3: 566-569.
- Nelson L, Hoffman R, Flomenbaum N, Goldfrank L, Howland MA. Goldfrank’s Toxicologic Emergencies. 9th edn. New York: McGraw-Hill; 2010.
- DeWitt CR, Waksman JC. Pharmacology, pathophysiology, and management of calcium channel blocker and beta blocker toxicity. Toxicol Rev. 2004, 23: 223-238.
- Mowry JB, Spyker DA, Brooks DE, McMillan N, Schauben JL. 2014 annual report of the American association of poison control centers’ national poison data system (NPDS): 32nd annual report. Clinical Toxicology. 2015; 53: 962-1147.
- Lheureux PE, S Zahir, M Gris, AS Derrey, A Penaloza. Bench-to-bedside review: hyperinsulinaemia/euglycaemia therapy in the management of overdose of calcium-channel blockers. Crit. Care. 2006; 10: 212.
- Devis G, Somers G, Ban Obberghan E, Malaisse WJ. Calcium antagonists and islet function: inhibition of insulin release by verapamil. Diabetes. 1975; 24: 247-251.
- Mégarbane B, Karyo S, Baud FJ. The role of insulin and glucose (hyperinsulinaemia/euglycaemia) therapy in acute calcium channel antagonist and beta-blocker poisoning. Toxicol Rev. 2004; 23: 215-222.
- Woodward C, Pourmand A, Mazer-Amirshahi, M. High dose insulin therapy, an evidence based approach to beta blocker/calcium channel blocker toxicity. DARU Journal of Pharmaceutical Sciences DARU. 2014; 22: 36.
- St-Onge M, Dube PA, Gosselin S, Guimont C, Godwin J, Archambault PM, et al. Treatment for calcium channel blocker poisoning: a systematic review. Clin Toxicol (Phila). 2014; 52: 926-944.
- Walter FG, G Frye, JT Mullen, BR Ekins, PA Khasigian. Amelioration of nifedipine poisoning associated with glucagon therapy. Ann Emerg Med. 1993; 22: 1234-1237.
- Kline JA, Leonova E, Raymond RM. Beneficial myocardial metabolic effects of insulin during verapamil toxicity in the anesthetized canine. Crit Care Ned. 1995; 23: 1251-1263.
- Engebretsen KM, Kaczmarek KM, Morgan J, Holger JS. High-dose insulin therapy in beta-blocker and calcium channel-blocker poisoning. Clin Toxicol (Phila). 2011; 49: 277-283.
- Shah SK, Goswami SK, Babu RV, Sharma G, Duarte AG. Management of Calcium Channel Antagonist Overdose with Hyperinsulinemia- Euglycemia Therapy: Case Series and Review of the Literature. Case Rep Crit Care. 2012; 2012: 927040.
- Burkes R, Wendorf G. A multifaceted approach to calcium channel blocker overdose: A case report and literature review. Clin Case Rep. 2015; 3: 566-569.