Case Report
Idiopathic Spinal Cord Herniation Incarcerated into a Bone Hole: A Case Report and Review of the Literature
Hisatsugu Kurimoto*, Toshikatu Mamada, Norihiko Nakata and Go Hayasaka
Department of Orthopedics, Teikyo University Hospital, Japan
*Corresponding author: Andrea Austin, Department of Emergency Medicine, Naval Medical Center San Diego, 34800 Bob Wilson Dr, San Diego, CA 92134, USA
Published: 24 Aug 2016
Cite this article as: Kurimoto H, Mamada T, Nakata N,
Hayasaka G. Idiopathic Spinal Cord
Herniation Incarcerated into a Bone
Hole: A Case Report and Review of the
Literature. Ann Clin Case Rep. 2016;
1: 1098.
Abstract
Background:Idiopathic spinal cord herniation (ISCH) was first reported in 1974 and is defined as a segment of spinal cord herniated through a ventral defect in the dura. Several reports have suggested
the pathogenesis, but it remains unclear. We report a very rare case of ISCH incarcerated in a
bone hole and suggest the pathogenesis was related to adhesions that resulted from a pre-existing
continuous leakage of cerebrospinal fluid (CSF) created by the bone defect or that the dura was
incarcerated in the pre-existing bone defect and adhesion between the dura and vertebra resulted in
the secondary dural defect.
A 77-year-old man presented with Brown- Séquardsyndrome below the T6 level. Magnetic
resonance imaging (MRI) showed that the thoracic spinal cord was displaced ventrally and partially
incarcerated into the vertebral body. There was no septum between the spinal cord and the dura
mater.
Intraoperatively, the dorsal dura mater was absent, and herniated spinal cord was identified after
durotomy. After releasing the cord herniation, the hiatus and the spinal cord were reduced.
Conclusions: The present case indicates the spinal cord herniation may have (1) been manufactured
by incarceration into a bone defect created by the flow of CSF through a pre-existing anterior dural
defect, (2) taken place after the incarceration of dura into the pre-existing bone defect, or (3) resulted
from adhesion between the dura and vertebra bereave the dura secondarily could be the underlying
pathogenesis of ISCH.
Keywords: Idiopathic spinal cord herniation (ISCH); Lumbar spinal cord stenosis (LCS)
Introduction
Wortzman et al. [1] first reported a case of idiopathic spinal cord herniation (ISCH) in 1974. In the years since magnetic resonance imaging (MRI) came into widespread use, the concept of ISCH has gradually become more frequently appreciated, and the number of reported cases has increased since 1990. This herniation exclusively occurs in the thoracic spine, typically between T4-T7 causing progressive myelopathy. Diagnosis is based on ventral displacement of the spinal cord observed on MRI and computed tomographic (CT) myelography. Nevertheless, the pathogenesis of ISCH has yet to be established. We report a case of ISCH with incarceration into a vertebral body and suggest two hypotheses.
Case Presentation
History and examination
A 77-year-old man presented with muscle weakness in his lower legs and difficulty walking,
which he began to notice at the age of 70. He consulted a physician and underwent lumbar surgery
at the age of 72 after being diagnosed with lumbar spinal cord stenosis (LCS). His symptoms did
not change. His lower leg muscles became weaker, and walking became increasingly difficult.
Neurological examination showed Brown-Séquard syndrome below the T8 level. Dyskinesia,
decreased sensation to pressure in the right lower leg and thermal nociception in the left lower leg
were seen. Muscle strength was reduced to 2/5 (unable to move joint against gravity) in both lower
legs. Deep tendon reflexes were exaggerated bilaterally at the knees and the ankles. His JOA score,
excluding the upper limb, was 4/11. Vesicorectal functions were preserved. No abnormalities were
seen on blood tests, biochemical tests, or chest X-rays.
Radiological findings
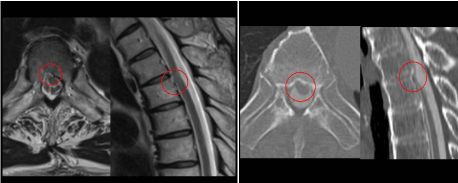
T2-weighted MRI showed that the spinal cord was displaced ventrally at T4/5 (Figure 1). CT myelography showed a hollow in the dorsal side of the T5 vertebra,and we suspected the presence of a bone defect and incarceration
of the spinal cord into the bone defect (Figure 1). From the clinical
history, physical findings, and images, we diagnosed him with
incomplete paresis due to spinal cord herniation and performed
surgery because his symptoms were progressive.
Operative findings
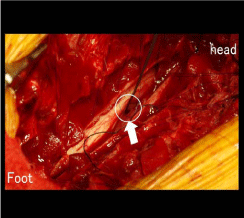
We performed laminectomy of T4-6. The dorsal dura was opened
at the T5 level, and the ventral dural defect and incarceration of the
spinal cord into the bone defect at the same level were identified
(Figure 2). No duplicate dura or adhesion between the spinal cord
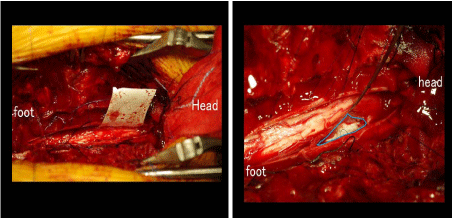
and dura was seen. After reduction of the cord herniation, the dural
defect was closed using artificial dura (Figure 3).
Postoperative course
The patient had temporary progression of muscle weakness, which
forced him to move around with a wheel chair, but the weakness
improved gradually. By 5 weeks after surgery, the patient regained
the ability to walk with a crutch and was discharged. Postoperative
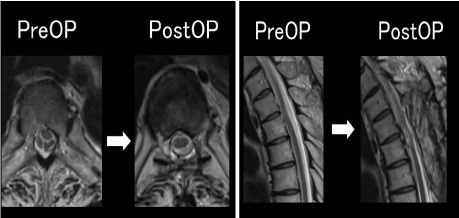
MRI showed the herniation at T4/5had disappeared, and the spinal
cord was reduced to its normal location (Figure 4). His JOA score,
excluding the upper limb, improved to 8/11 at 1 year after surgery.
Figure 1
Figure 1
T2-weighted MRI showed that the spinal cord was displaced
ventrally at T4/5. CT myelography showed a hollow in the dorsal side
of the T5 vertebra, and we suspected the presence of a bone defect and
incarceration of the spinal cord into the bone defect.
Figure 2
Figure 2
The dorsal dura was opened at the T5 level, and the ventral dural
defect and incarceration of the spinal cord into the bone defect at the same
level were identified.
Discussion
Spinal cord herniation is defined as a segment of spinal cordherniated through a ventral defect in the dura and often presents with Brown-Séquard syndrome, but patients present with paraplegia
rather than hemiplegia. The pathogenesis is still unclear, but Summers
et al. [2] classified the herniation under “congenital dural deficiency”
“history of trauma” “pressure erosion of dura” and “duplication of
ventral dura” [1]. Najjar et al. [3] and Barrenecheaet et al. [4] reported cases of spinal cord herniation with no duplicated dura, similar to the
present case [3-7]. Our patient had no history of trauma or duplicated
dura, and we presumed the pathogenesis was related to either a
“congenital dural deficiency” or “pressure erosion of the dura”
Either way; patients need to undergo surgical intervention because
symptoms such as muscle weakness progress gradually.
In approximately 140 articles that we reviewed, seven cases
involved incarceration into bone defects, and only reported multiple
cases in one review Imagama et al. [5]. In our case, the positions of
the dural defect and bone defect were within the same vertebra at the
same level, so it is unlikely that the position was just a coincidence.
However, even the reviews that reported bone defects did not discuss
or propose a pathogenic mechanism, and the etiology remains
unknown.
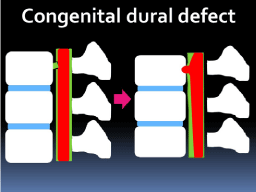
We proposed two hypotheses for the formation of the bone defect.
The first hypothesis was that a continuous diapedesis of CSF through
the congenital dural defect created the bone defect and forced the
spinal cord to incarcerate and adhere. As a result, the spinal cord
herniation was formed (Figure 5).
maintain that 60% of thoracic spinal cord herniations are seen
at T4-6 Muraoka et al. [6]. Imagama et al. [5] did not mention
the vertebral level with the bone defect; it remains unclear if bone
defects are likely to be seen in T4-6, but judging from the anatomical, alignment of the thoracic vertebra (kyphotic), the flow of CSF is
slower on the dorsal side, resulting in the storage and turbulence of
CSF. These mechanisms are most likely to occur at T4-6, suggesting
that formation of a bone defect process may be more likely here.
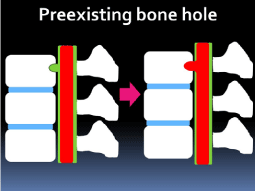
The second hypothesis is that the dura mater was incarcerated
into the pre-existing bone hole, and the adhesion that occurred
between the dura and vertebra resulted in the secondary dural defect
and spinal cord herniation (Figure 6). Pre-existing bone defects might
result from the scalloping of a herniated disc or a hematoma or may
occur developmentally during the prenatal period. Barrenecheaet al.
[4] guessed that arachnoid herniation may occur through an anterior
dural defect into the extradural space and CSF would flow freely in
and out. In time, as the spinal cord develops adhesions to the edges
of the dural defect, CSF flow is impeded. Cardiac pulsations and
respiratory movements then push the cord into the defect and cause
the herniation [4].
Najjar et al. [3] took into consideration another hypothesis. An
inflammatory process involving the spinal cord and/or the meninges
is the initial event that leads to ventral adhesion of the cord to the
dura. The spinal cord is gradually tethered and later herniated through
a dural defect probably caused by the inflammation, adhesion, and
pulsatility of the now fixed spinal cord [3]. The theory held by Najjar
et al. [3] does not explain the coincidence of the close proximity of the
bone defects to the dural defects and does not support the conclusion
that the existence of pre-existing bone defects led to the formation of
spinal cord herniation. Judging from time series, the first hypothesis
advocating dural defects preceding bone defects seemed to affirm
Barrenechea et al. [4] but further research is needed. In this case,
we suspect the spinal cord herniation took place as a result of the
entrapment of the spinal cord by incarceration into the acquired bone
hole, which was probably made by the continuous leakage of CSF
through the congenital dural defect, and resulted in the myelopathy.
Figure 3
Figure 4
Figure 4
Postoperative MRI showed the herniation at T4/5had disappeared,
and the spinal cord was reduced to its normal location.
Figure 5
Figure 6
Figure 6
The second hypothesis is that the dura mater was incarcerated
into the pre-existing bone hole, and the adhesion that occurred between the
dura and vertebra resulted in the secondary dural defect and spinal cord herniation.
Conclusion
We report a case of spinal cord herniation with slow progression of myelopathy and the underlying cause was determined to be incarceration into the bone hole. We assumed that the spinal cord herniation more likely developed as a result of the incarceration into the bone defect made by the flow of CSF through the pre-existing anterior dural defect than for the spinal cord to have herniated after incarceration of the dura into the pre-existing bone defect and that adhesion between the dura and vertebra bereave the dura secondarily can be the pathogenesis of ISCH.
References
- Wortzman G, Tasker RR, Rewcastle NB, Richardson JC, Pearson FG. Spontaneous incarcerated herniation of the spinal cord into a vertebral body. a unique case of paraplegia. J Neurosurg. 1974; 41: 631-635.
- Summers Johanne C, Balasubramani V Yagnesh, Chan Patrick, Jeffrey V.Rosenfeld. Idiopathic spinal cord herniation: clinical review and report of three cases. Asian J Neurosurg. 2013; 8: 97-105.
- Najjar NW, Baeesa SS, Lingawiss. Idiopathic spinal cord herniation. A new theory of pathogenesis. SurgNeurol. 2004; 62: 161-171.
- Barrenechea IJ, Lesser JB, Gidekel AL, Turjanski L, Perin NI. Diagnosis and treatment of spinal cord herniation: a combined experience. J Neurosurg Spine. 2006; 5: 294-302.
- Imagama S, Matsuyama Y, Sakai Y, Nakamura H, Katayama Y, Ito Z, et al. Image classification of idiopathic spinal cord herniation based on symptom severity and surgical outcome: a multicenter study. J Neurosurg Spine. 2009; 11: 310-319.
- Muraoka S, Shimizu K, Nakamura E, et al. Idiopathic spinal cord herniation: a case report and meta-analysis. Orthop Surg and Traumatol. 2012; 55: 101-105.
- Masuzawa H, Nakayama H, Shirata N, Takeyo Suzuki. Spinal cord herniation into a congenital extradural arachnoid cyst causing Brown- Séquard syndrome Case report. J Neurosurg. 1981; 55: 983-986.