Case Report
Common and Uncommon Complications of Autologous Pubovaginal Sling
Andraska E, Santiago-Lastra Y and Stoffel JT*
Department of Urology, University of Michigan Medical School, USA
*Corresponding author: John T. Stoffel, Department of Urology, University of Michigan Medical School, Taubman Center Floor 2 Reception C, 1500 E Medical Center Dr SPC, 5330, Ann Arbor MI 48109, USA
Published: 18 Jul, 2016
Cite this article as: Andraska E, Santiago-Lastra Y,
Stoffel JT. Common and Uncommon
Complications of Autologous
Pubovaginal Sling. Ann Clin Case Rep.
2016; 1: 1055.
Abstract
Introduction: Erosion of autologous pubovaginal slings, placed for treatment of urinary
stress incontinence, is uncommon. The complication profile of autologous slings differs from
polypropylene midurethral slings.
Materials: This patient presented with supra pubic pain, dysuria, recurrent urinary tract infections/
pyelonephritis, stress incontinence, and urinary retention requiring intermittent catheterization.
Cystoscopy revealed that her previous pubovaginal sling, placed 4 months prior, had perforated the
bladder was responsible for the symptoms.
Results: The patient required a cystectomy and urinary diversion to treat her refractory symptoms.
Compared to midurethral polypropylene slings, autologous pubovaginal slings have a much lower
incidence of material extrusion or erosion but a higher incidence of infectious complications,
particularly wound infections.
Conclusions: Erosion of pubovaginal sling into the bladder is an uncommon source of refractory
urinary symptoms.
Keywords: Complications; Autologous pubovaginal sling; Erosion
Introduction
Stress urinary incontinence (SUI) is a common problem, affecting up to 40% of US women [1]. Autologous fascial pubovaginal slings (PVS) represent an effective option for surgical treatment of SUI and are rarely associated with some of the complications that are seen with synthetic midurethral sling (MUS) placement, such as mesh erosion, extrusion or material infection. However, autologous sling procedures are not without risk for complications and while common complications of PVS such as urinary disturbances (retention, slow stream) and urinary tract infection (UTI) are well documented in the literature, there is insufficient data on the more uncommon complications, such as extrusion of the sling and visceral perforation. Here, we present a case of intravesical erosion of an PVS and review the literature on common and uncommon complications of PVS.
Case Report
Presenting problem
A 69-year-old woman presented with suprapubic pain, dysuria, recurrent urinary tract infections/
pyelonephritis, stress incontinence, and urinary retention requiring intermittent catheterization.
She had a previous history of autologous PVS for treatment of stress incontinence 4 months prior to
evaluation and had recently been treated with sacrospinous ligament fixation (SSLF) vaginal vault
reconstruction. During the SSLF surgery, intra operative cystoscopy demonstrated a calcified band
of tissue between the trigone and the superior bladder neck. Visual inspection of the material during
cystoscopy revealed it to be the previous autologous PVS which appeared to perforate the bladder
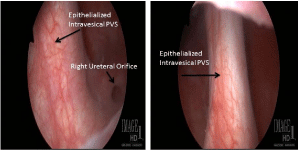
at the right bladder neck and exit the bladder through the trigone, near the ureteral orifice. The
right ureteral orifice was widely patulous and scarred in an open position (Figure 1). She underwent
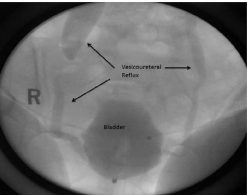
fluoroscopic urodynamic evaluation, which demonstrated severe bilateral VUR (grade 5 on right,
grade 3 on left) at low volumes (Figure 2), a 160ml functional bladder capacity, stress urinary
incontinence with a valsalva leak point pressure less than 50 cm H2O, and an atonic detrusor. Renal
ultrasound showed bilateral hydroureteronephrosis, consistent with reflux.
Treatment and intervention
Given the combination of small bladder capacity, severe continuous stress incontinence and symptomatic vesicoureteral reflux, the patient was first presented the option of bilateral ureteral reimplantation with augmentation cystoplasty and repeat autologous PVS. However the patient was extremely frustrated with her current symptoms and did not wish to pursue intermittent catheterization as a possible a long term emptying option. As an alternative, the patient elected to undergo cystectomy with urinary diversion.
During the surgery, cystoscopy was first performed and the intravesical portion autologous PVS was identified. Both ureteral orifices were noted to be widely patent which was consistent with the reflux observed on previous voiding cystourethrogram. A midline laparotomy incision was then used, and a small, contracted bladder was identified in the space of Retzius, adherent to the pubic symphysis. A cystotomy was next performed to confirm the position of the eroded sling and, as noted on cystoscopy (Figure 3). A simple cystectomy was performed with resection of the bladder to the proximal urethra, the retropubic part of the sling was removed, and the urethral stump was closed. After the cystectomy was completed, ureteral dissection was performed and a standard ileal conduit urinary diversion was created. The patient’s postoperative course was notable for a postoperative ileus, but her recovery was otherwise uncomplicated. She experienced no further complication. At her six month follow up clinic appointment, she reported that her urinary tract infections/pyelonephritis has resolved and her urinary quality of life is improved.
Figure 1
Figure 2
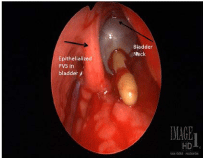
Figure 3
Figure 3
View of sling from open cystotomy. Bladder neck is identified by catheter balloon and epithelialized sling is located to left of bladder neck.
Review of the Literature
Outcomes
Global cure rates for AF-PVS range from 67-97% [1-4]. These are measured using objective measures to determine change in volume of stress incontinence, such as pad weight and pad number, with cure many times cited as patient reported pad use of <1 pad per day. Subjective cure rates, which focus on patient/provider perception of improvement, are also quantified using pain scores, quality of life scoring systems, and patient-reported satisfaction rates range from 51-86% [1-3,5].
Complications
Reported global complication rate for AF-PVS range from 14% to 29% [1,5] but the true complication rate for PVS is difficult to compare across series due to variability in defining success and lack of reporting adverse advents. Since our patient displayed complications of organ injury from the PVS, urinary tract infections, and urinary incontinence, reviewed we evaluated three major categories of AF-PVS complications, Injury to surrounding organs, infection, and voiding dysfunction, to give context to the incidence of PVS related complications
Injury
There are very few cases of erosion of autologous tissue, and most of these cases involve either the vagina or the urethra. There are only two documented cases of bladder erosion, specifically following AF-PVS operations [6-12]. Athanasopoulos et al. [5] report vaginal extrusion of the sling in two patients (0.08% incidence in author’s series). Erosion into the urethra has also been reported in 4 additional patients, including 3 occurring de novo after surgery and one after urethral injury during intermittent catheterization [9-12]. In comparison, the incidence of material erosion for patients is 1-5% of patients undergoing MUS. Of patients requiring post MUS surgical intervention, about 34% are related to mesh erosion or extrusion, with vaginal extrusion being the most common [13,14].
Bladder associated injury during MUS surgery has been reported to be between 0-7% [15], although care must be used in interpreting aggregate statistics since mesh type, location, and placement technique may alter incidence of complication for a specific MUS sling type. Similar injury rates have been reported in PVS series [16]. In a prospective study evaluating three multicenter clinical trials, universal intra operative cystoscopy at incontinence surgery detected a lower urinary tract abnormality in 5.2% of PVS cases. Of which, 76% were likely related to sling placement. Risk factors for intra operative bladder injury included patient age and smoking status. Trainee participation was not associated with an increased risk of injury [15].
Although our patient required a cystectomy for her severe stress incontience, vesicoureteal reflux, and small bladder capacity, many intravesical sling complications, both mid urethral and PVS, can be managed endoscopically. Patients with material extrusion into the bladder typically present with pelvic pain, dysuria, and recurrent UTIs usually which prompt evaluation and diagnosis [9]. The most conservative management for such cases is removal of the intravesical material using cystoscopic identification and excision. In a study evaluating urinary tract erosions after synthetic pubovaginal sling placement, Clemens et al. [6] reported complete relief of pain and incontinence following removal of intravesical contents with cystoscopic guidance. More invasive approaches can be considered when endoscopic excision is not indicated or not successful, as with Misrai et al. [7] report of a cohort of 75 patients who underwent complete surgical resection of a synthetic suburethral sling following intravesical erosion. In this series, all patients had improvement of pain and urinary urgency, while recurrent incontinence developed in 52% of patients.
Infection
Genitourinary infections are a common complication of AF-PVS. Infectious cystitis represents a substantial burden for patients undergoing the procedure, affecting up to 92% of patients, and a subset of patients develop pyelonephritis (2%) [3]. Urinary retention after AF-PFS likely increases the risk of UTI. Wound-associated infections incidence has been reported to be as high to 22% for PVS patients and reoperation secondary to wound complications has been reported in up to 3.4% of patients who undergo AF-PVS [1-3,5]. This contrasts sharply with MUS, where a recent decision analysis suggested that peri-operative antibiotics may not be necessary given the overall low rate and re-operation due to wound infections is extremely uncommon [8].
Voiding symptoms
One of the most common complications involves persistent or de novo urge incontinence following surgery. Persistent postoperative urge incontinence has been reported in 10% to 33% of PVS cases, with de novo urge incontinence comprising 3% to 30% of patients [1-3,5]. This can often be corrected with anticholinergic medication.
Another common problem following AF-PVS involves postoperative urinary retention, with an incidence from 2% to 12% [5,10]. While this resolves in most patients, there is a subset of patients between 2% and 7% of patients with urinary retention who require long-term intermittent catheterization [1,10].This is not dissimilar to reported 0-18% retention rates for mid urethral slings and non-autologous slings. However, these adverse events are less likely to require operative intervention when compared to AF-PVS [1,3].
Most PVS voiding related adverse events can be managed conservatively with observation and medication but some patients requiring additional interventions such as urethrolysis or sling division. PVS sling revision may result in subsequent return of stress incontinence symptoms which can then be treated with additional sling surgery or injection of bulking agents. Reoperations secondary to voiding dysfunction may arise with 1.9% to 6% of patients, and [3,5].
Conclusion
We discuss a case of bladder erosion of AF-PVS and subsequent surgical repair with cystectomy, an aggressive approach given the significant morbidity following the patient’s erosion. AF-PVS is an effective procedure for treating stress incontinence, but the procedure still carries a potential for significant complication, albeit uncommon. Our patient and review of the literature demonstrates that PVS can cause organ injury, chronic infection, and voiding dysfunction. When compared generally to MUS procedures, AF-PVS has a lower incidence of erosion but a higher incidence of infectious complications, particularly wound infections. Voiding dysfunction is similar in both sling types and re-operation is occasionally needed to treat urinary retention. Although uncommon, PVS complications and potential outcomes should be reviewed with patients during operative planning.
References
- Parker W, Gomelsky A, Padmanabhan P. Autologous fascia pubovaginal slings after prior synthetic anti-incontinence procedures for recurrent incontinence: A multi-institutional prospective comparative analysis to de novo autologous slings assessing objective and subjective cure. Neurourology and urodynamics. 2015; 35: 604-608.
- Welk B, Herschorn S. The autologous fascia pubovaginal sling for complicated female stress incontinence. Canadian urological association journal. 2012; 6: 36-40.
- Albo ME, Richter HE, Brubaker L, Norton P, Kraus SR, Zimmern PE, et al. Burch colposuspension versus fascial sling to reduce urinary stress incontinence. N Engl J Med. 2007; 356: 2143-2155.
- Chou EC, Flisser AJ, Panagopoulos G, Blaivas JG. Effective treatment for mixed urinary incontinence with a pubovaginal sling. J Urol. 2003; 170: 494-497.
- Athanasopoulos A, Gyftopoulous K, McGuire E. Efficacy and preoperative prognostic factors of autologous fascia rectus sling for treatment of female stress urinary incontinence. Urology. 2011; 78:1038-1039.
- Clemens J, Delancy J, Faerber G, Westney O, McGuire E. Urinary tract erosions after synthetic pubovaginal slings: diagnosis and management strategy. Urology. 2000; 56: 589-594.
- Misrai V, Roupret M, Xylinas E, Cour F, Vaessen C, Haertig A, et al. Surgical resection for suburethral sling complications after treatment for stress urinary incontinence. Journal of Urology. 2009; 81: 2198-2202.
- Shepherd JP JK, Harmanli O. Is antibiotic prophylaxis necessary before midurethral sling procedures for female stress incontinence? A decision analysis. Int Urogynecol J. 2014; 25: 227-233.
- Amundsen CL, Flynn BJ, Webster GD. Urethral erosion after synthetic and nonsynthetic pubovaginal slings: differences in management and continence outcome. J Urol. 2003; 170: 134-137.
- Webster TM, Gerridzen RG. Urethral erosion following autologous rectus fascial pubovaginal sling. Can J Urol. 2003; 10: 2068-2069.
- Handa VL, Stone A. Erosion of a fascial sling into the urethra. Urology. 1999; 54: 923.
- Golomb J, Groutz A, Mor Y, Leibovitch I, Ramon J. Management of urethral erosion caused by a pubovaginal fascial sling. Urology. 2001; 57: 159-160.
- Ordorica R, Rodriguez AR, Coste-Delvecchio F, Hoffman M, Lockhart J. Disabling complications with slings for managing female stress urinary incontinence. BJU Int. 2008; 102: 333-336.
- Morgan TO, Westney OL, McGuire EJ. Pubovaginal sling: 4-YEAR outcome analysis and quality of life assessment. J Urol. 2000; 163: 1845-1848.
- Zyczynski HM, Sirls LT, Greer WJ, Rahn DD, Casiano E, Norton P, et al . Findings of universal cystoscopy at incontinence surgery and their sequelae. Am J Obstet Gynecol. 2014; 210: 480.
- Schimpf MO, Rahn DD, Wheeler TL, Patel M, White AB, Orejuela FJ, et al. Sling surgery for stress urinary incontinence in women: a systematic review and metaanalysis. American Journal of Obstetrics and Gynecology. 2014; 211: 71.