Case Report
The Potential Role of 18F-FDG PET in the Early Detection of Basal Ganglia Germinoma in a 13-Year Old Patient with Diabetes Insipidus
Vali R1*, Shammas A2, Khazaee A2, Widjaja E3 and Bouffet E3
1Division of Nuclear Medicine, University of Toronto, Canada
2Department of Diagnostic Imaging, University of Toronto, Canada
3Department of Pediatric Hematology and Oncology, University of Toronto, Canada
*Corresponding author: Reza Vali, The hospital for Sick children, University of Toronto, Toronto, On. Canada
Published: 10 Jul, 2016
Cite this article as: Vali R, Shammas A, Khazaee A,
Widjaja E, Bouffet E. The Potential
Role of 18F-FDG PET in the Early
Detection of Basal Ganglia Germinoma
in a 13-Year Old Patient with Diabetes
Insipidus. Ann Clin Case Rep. 2016; 1:
1052.
Abstract
Central nervous system (CNS) germ cell tumors (GCT) are a heterogeneous group of tumors arising predominantly in the midline structures of the pineal and/or suprasellar regions. Approximately, less than 14% of all intracranial GCTs occur in the basal ganglia or thalamus. It has been reported that a remarkable number of these patients experience a delay in time to diagnosis which may result in significant consequences. Therefore, to prevent this delay, introducing a non-invasive imaging modality with the ability of early detection of CNS-GCT is of pivotal importance. This case report signifies the potential role of 18F-FDG PET scan in early diagnosis of a patient with Basal Ganglia Germ Cell Tumor, while other imaging studies were not confirmatory.
Keywords: Brain neoplasms; Germinoma; Basal ganglia disease; Positron emission tomography; Child
Abbreviations: CNS: Central Nervous System; GCT: Germ Cell Tumors; MRI: Magnetic Resonance Imaging; CT: Computed Tomography; PET: Positron Emission Tomography; 18F-FDG: 18F-Fluorodeoxyglucose; MET: 11C-Methionine; SUVmax: Maximum Standardized Uptake Value
Introduction
Central nervous system (CNS) germ cell tumors (GCT) are a heterogeneous group of tumors
arising predominantly in the midline structures of the pineal and/or suprasellar regions [1]. The
diagnosis of GCTs is based on clinical signs and symptoms, tumor markers, imaging, cytological
cerebrospinal fluid (CSF) evaluation, and histologic confirmation. Computed tomography (CT) and
magnetic resonance imaging (MRI) are commonly used in detecting GCTs. It has been reported
that a significant number of patients with GCTs experience a delay in time to diagnosis, in some
cases despite evaluation by general pediatrician and specialists [2]. Such delays may have significant
consequences, in particular in term of endocrine deficit, neurologic impairment and increased risk
of disseminated disease [2-4]. Therefore, the importance of the early diagnosis of GCT in patients
is paramount.
Positron emission tomography (PET) has been shown to be a promising functional imaging
modality in detecting a neoplastic processes, and evaluation of response to therapy [5]. Combining
PET system with CT scan or MRI provides registered functional and anatomical images, which
are of value in oncology [6]. Different PET tracers including 18F-fluorodeoxyglucose (18F-FDG),
11C-methionine (MET), and 11C-choline have been used in neuro-oncology for the evaluation of
brain tumors such as glioma, and germinoma [3,7,8]. Few reports suggest using MET or 18F-FDG
PET in CNS germinoma.
We herein describe a 13-year-old female patient with a germinoma of the basal ganglia who
presented with diabetes insipidus, headache, nausea and vomiting. MRI showed thickening of
the pituitary stalk, but no definite abnormality was detected elsewhere. Further investigation with
18F-FDG PET demonstrated asymmetric reduced activity in the basal ganglia, which was suggestive
of germinoma. The possibility of suprasellar germinoma was further suspected on follow-up MRI
that demonstrated progression of the lesion in the suprasellar region. An endoscopic biopsy was subsequently performed and confirmed the diagnosis.
Case Presentation
A 13-year-old female with one-year history of diabetes insipidus presented with nausea and vomiting, and right-sided headache migrating to the back. She did not have any complain of diplopia and her peripheral vision testing was normal. She reported a weight loss of 14 lbs during the last months as well as polydipsia, nocturia and hair loss. During her initial visit, neurologic examination including cranial nerves and cerebellar function were normal. She was at breast Tanner stage 2 and Pubic hair Tanner stage 3. A water deprivation test confirmed the diagnosis of diabetes insipidus. Serum alpha-fetoprotein was reported 1 microgram/L (normal range 1-4 microgram/L). Serum B-human chorionic gonadotropin (B-HCG) was reported <1 IU/L (normal range < 1.2 IU/L). Further investigation with MRI showed pituitary stalk thickening and absent normal T1 signal of the posterior pituitary gland with no definite abnormality in the basal ganglia (Figure 1A and B). CSF evaluation was negative for malignancy and tumor markers were negative in the CSF.
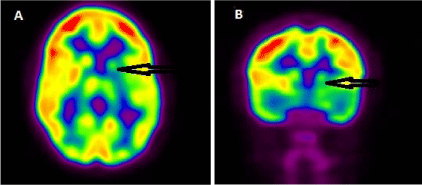
A PET scan with 18F-FDG and skeletal survey were performed to assess for possible diagnosis of histiocytosis. No definite lytic lesion was detected on the skeletal survey. However, an asymmetric activity was detected in the basal ganglia without any extracranial abnormality (Figure 2). There was relative hypometabolism in the left basal ganglia with a maximum standardized uptake value (SUVmax) of 5.8 compared with the contralateral basal ganglia with an SUVmax of 9.1. The ipsilateral cerebral cortex also showed relatively decreased 18F-FDG activity compared with the contralateral cerebral cortex. The PET finding was interpreted as suspicious for left basal ganglia tumoral involvement.
A repeat MRI after three months showed slight increase in the thickness of the pituitary stalk. The posterior pituitary bright spot remained absent. There was high T2/FLAIR signal in the superior aspect of the left basal ganglia and anterior limb of the internal capsule associated with a focus of enhancement and volume loss in the left basal ganglia suspicious for germinoma (Figure 1C and D). Following blood works didn’t reveal any remarkable changes; alpha-feto protein was reported 2 micrograms/L (normal range 1-4 micrograms/L) and B-HCG was reported less than 1 IU/L (normal range < 1.2 IU/L).
CSF alpha feto protein was below 1 microgram/L and CSF B-HCG was reported less than 1 IU/L, both were insignificant upon hospital reference values. Ultimately, the diagnosis of suprasellar germinoma was confirmed by biopsy. The patient was treated with 4 courses of carboplatin-etoposide. Follow-up MRI after the end of chemotherapy showed significant improvement in pituitary stalk thickening, slight improvement in the abnormal signal in the superior aspect of the left basal ganglia and deep white matter. The patient eventually received whole brain radiation at a dose of 24 Gy in 13 sessions.
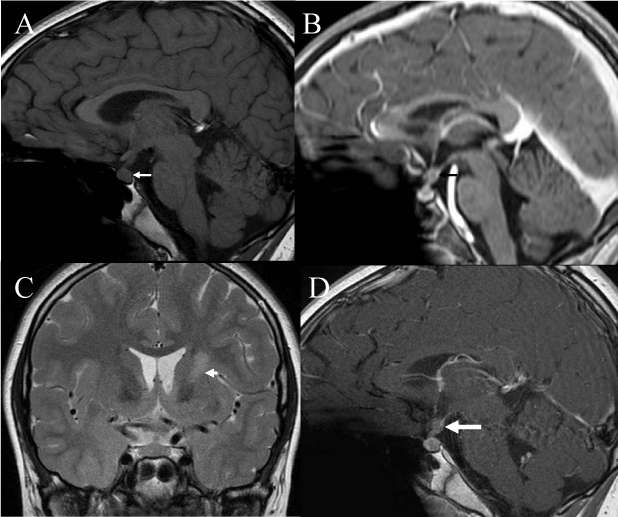
Figure 1
Figure 1
(A) Pre and (B) post contrast sagital T1 weighted imaging at presentation demonstrate absent normal high T1 hyperintensity of the posterior pituitary gland and post contrast scan shows pituitary stalk thickening, but no definite abnormality in the basal ganglia (not shown). (C) contrast sagital T2 and (D) post contrast T1 weighted imaging three months later demonstrate high T2 signal in the left basal ganglia and left anterior limb of internal capsule and further thickening of the pituitary stalk (arrow).
Figure 2
Figure 2
PET scan displayed in axial (A) and coronal (B) views showed asymmetric activity in the basal ganglia, as well as relative hypometabolism in the left basal ganglia. The ipsilateral cerebral cortex also showed relatively decreased [18] F-FDG activity compared with the contralateral cerebral cortex. The PET finding was interpreted as suspicious for left basal ganglia tumoral involvement.
Discussion
Central nervous system GCTs are divided into germinomas (approximately 50%–70% of cases) and non-germinomatous germ cell tumors according to their clinicopathologic features [9]. These tumors are primarily seen in children accounting for 3-5% of pediatric brain tumors with a peak age of 10-12 years [9-12]. The most common primary tumor sites are the pineal region, the suprasellar area and the basal ganglia. Approximately, less than 14% of all intracranial GCTs occur in the basal ganglia or thalamus [13]. Initial symptoms are related to the tumor location. For suprasellar tumors, earliest symptoms usually involve endocrine dysfunction, most frequently diabetes insipidus and associating polyuria and polydipsia. Eventually other endocrine manifestations such as growth impairment or arrest, delayed or precocious puberty, hypothyroidism, and visual disturbances may occur as the tumor grows dorsally toward the optic chiasm. Patients with pineal tumor present with visual symptoms (parinaud syndrome) and signs and symptoms of increased intracranial pressure (ICP) [10,14]. Progressive hemiparesis is the most common presenting symptom of basal ganglia tumors. Patients diagnosed with suprasellar and basal ganglia germ cell tumors may have a long prodrome, up to several years in duration [1]. This is a report in which 18F-FDG PET suggested tumoral involvement in the left basal ganglia before the MRI findings were visualized and may suggest a potential role for PET scan in early detection of suprasellar germinoma.
MRI is a sensitive imaging to identify suprasellar GCTs [12]. However, MRI cannot differentiate different types of GCTs and except in cases where characteristic serum and/or CSF tumor marker is elevated, biopsy is needed for definite diagnosis [12]. Both the clinical manifestations and MRI findings may progress for months before a definitive diagnosis is made. Several distinct MRI findings are described in the literature for basal ganglia germinoma, ranging from subtle non-enhancing patchy lesions to enhancing huge masses [15-17]. Phi et al. [17] classified these findings into four types. Type 1 lesions show minimal or no enhancement, no mass effect and may be missed for long period of time or mistaken for benign lesions. Since the diagnosis is more delayed in this group, hemiparesis usually progress into profound motor deficit [17]. An imaging modality with the potential ability in early detection of GCTs is crucial to select the patients who need further investigation with biopsy. Functional imaging such as PET scan may play a role in this respect. Functional images may detect tumoral involvement weeks or months before any detectable structural abnormality is identified.
PET scan with 18F-FDG, 11C-MET, or 11C-choline has been shown to be useful in detection of brain glioma and germinoma [3,7,8,18]. PET scan can be useful when there is a suspicious lesion on MRI but the biopsy cannot be performed because of the location of the lesion or because of other clinical conditions [19]. In our case 18F-FDG PET showed hypometabolism in the involved basal ganglia as well as relatively decreased 18F-FDG activity in the ipsilateral cerebral cortex compared with the contralateral cerebral cortex. The exact mechanism of reduced metabolic activity in the ipsilateral cerebral cortex is not clear. However, this finding has been described before [17]. A few reports are available describing 18F-FDG PET findings in basal ganglia. In those studies, basal ganglia germinomas showed a relatively reduced 18F-FDG activity compared to normal basal ganglia, regardless of the types of germinomas on MRI [17,20]. However, to our knowledge, there is no report describing the possibility for 18F-FDG PET to suggest the diagnosis of basal ganglia germinoma before definitive findings appear on MRI. This is of pivotal importance in cases of suprasellar and basal ganglia germinoma in which the diagnosis may be delayed in spite of a thorough investigation. Since early diagnosis can prevent the disease from progression and have a commendable impact on the outcome, further evaluation with PET scan may be considered in specific clinical cases. Further prospective studies are needed to clarify the exact role of the PET scan compared to MRI in detecting all kinds of GCTs in the early stages of the disease.
References
- Kliegman R, Nelson WE. Nelson textbook of pediatrics. 19th ed. Philadelphia, PA: Elsevier/Saunders; 2011.
- Sethi RV, Marino R, Niemierko A, Tarbell NJ, Yock TI. MacDonald SM. Delayed diagnosis in children with intracranial germ cell tumors. The Journal of pediatrics. 2013; 163: 1448-1453.
- Sudo A, Shiga T, Okajima M, Takano K, Terae S, Sawamura Y, et al. High uptake on 11C-methionine positron emission tomographic scan of basal ganglia germinoma with cerebral hemiatrophy. AJNR American journal of neuroradiology. 2003; 24: 1909-1911.
- Ramelli GP, von der Weid N, Stanga Z, Mullis PE, Buergi U. Suprasellar germinomas in childhood and adolescence: diagnostic pitfalls. Journal of pediatric endocrinology & metabolism. JPEM. 1998; 11: 693-697.
- Zhu A, Lee D, Shim H. Metabolic positron emission tomography imaging in cancer detection and therapy response. Seminars in oncology. 2011; 38: 55-69.
- Nelson SJ, Day MR, Buffone PJ, Wald LL, Budinger TF, Hawkins R, et al. Alignment of volume MR images and high resolution [18F]fluorodeoxyglucose PET images for the evaluation of patients with brain tumors. Journal of computer assisted tomography. 1997; 21: 183-191.
- Kawai N, Miyake K, Nishiyama Y, Yamamoto Y, Miki A, Haba R, et al. Targeting optimal biopsy location in basal ganglia germinoma using (11)C-methionine positron emission tomography. Surgical neurology. 2008; 70: 408-413.
- Tan H, Chen L, Guan Y, Lin X. Comparison of MRI, F-18 FDG, and 11C-choline PET/CT for their potentials in differentiating brain tumor recurrence from brain tumor necrosis following radiotherapy. Clinical nuclear medicine. 2011; 36: 978-981.
- Jennings MT, Gelman R, Hochberg F. Intracranial germ-cell tumors: natural history and pathogenesis. Journal of neurosurgery. 1985; 63: 155-167.
- Hoffman HJ, Otsubo H, Hendrick EB, Humphreys RP, Drake JM, Becker LE, et al. Intracranial germ-cell tumors in children. Journal of neurosurgery. 1991; 74: 545-551.
- Lin IJ, Shu SG, Chu HY, Chi CS. Primary intracranial germ-cell tumor in children. Zhonghua yi xue za zhi = Chinese medical journal. Free China ed. 1997; 60: 259-264.
- Echevarria ME, Fangusaro J, Goldman S. Pediatric central nervous system germ cell tumors: a review. The oncologist. 2008; 13: 690-699.
- Tamaki N, Lin T, Shirataki K, Hosoda K, Kurata H, Matsumoto S, et al. Germ cell tumors of the thalamus and the basal ganglia. Child's nervous system: ChNS: official journal of the International Society for Pediatric Neurosurgery. 1990; 6: 3-7.
- Goodwin TL, Sainani K, Fisher PG. Incidence patterns of central nervous system germ cell tumors: a SEER Study. Journal of pediatric hematology/oncology. 2009; 31: 541-544.
- Moon WK, Chang KH, Kim IO, Han MH, Choi CG, Suh DC, et al. Germinomas of the basal ganglia and thalamus: MR findings and a comparison between MR and CT. AJR American journal of roentgenology. 1994; 162: 1413-1417.
- Okamoto K, Ito J, Ishikawa K, Morii K, Yamada M, Takahashi N, et al. Atrophy of the basal ganglia as the initial diagnostic sign of germinoma in the basal ganglia. Neuroradiology. 2002; 44: 389-394.
- Phi JH, Cho BK, Kim SK, Paeng JC, Kim IO, Kim IH, et al. Germinomas in the basal ganglia: magnetic resonance imaging classification and the prognosis. Journal of neuro-oncology. 2010; 99: 227-236.
- Lee J, Lee BL, Yoo KH, Sung KW, Koo HH, Lee SJ, et al. Atypical basal ganglia germinoma presenting as cerebral hemiatrophy: diagnosis and follow-up with 11C-methionine positron emission tomography. Child's nervous system: ChNS: official journal of the International Society for Pediatric Neurosurgery. 2009; 25: 29-37.
- Rupp D, Molitch M. Pituitary stalk lesions. Current opinion in endocrinology, diabetes, and obesity. 2008; 15: 339-345.
- Okochi Y, Nihashi T, Fujii M, Kato K, Okada Y, Ando Y et al. Clinical use of 11 C-methionine and 18 F-FDG-PET for germinoma in central nervous system. Annals of nuclear medicine. 2014; 28: 94-102.