Case Report
Terlipressin-Induced Irreversible Severe Skin Necrosis: A Dreadful Complication
Cleva RD, Santarém OLDA and Herman P*
Department of Gastroenterology, University of São Paulo Medical School, Brazil
*Corresponding author: Paulo Herman, Department of Gastroenterology, University of São Paulo Medical School, Praça Santos Coimbra 10, São Paulo CEP 05614- 050, Brazil
Published: 07 Jul, 2016
Cite this article as: Cleva RD, Santarém OLDA, Herman P.
Terlipressin-Induced Irreversible Severe
Skin Necrosis: A Dreadful Complication.
Ann Clin Case Rep. 2016; 1: 1040.
Abstract
Terlipressin (triglycyl lysine vasopressin), a synthetic analogue of vasopressin, has been employed in the last years for the treatment of upper digestive bleding from esophagogastric varices and for hepatorenal syndrome. Several adverse effects have been reported, all reversible after dose reduction or drug interruption. The authors report two rare cases of irreversible severe skin necrosis induced by terlipressin infusion.
Keywords: Terlipressin; Portal hypertension; Digestive bleeding; Hepatorenal syndrome
Introduction
Esophageal variceal bleeding (EVBEVB occurs in 10–20% of cirrhotic patients per year and each
bleeding episode is associated with a 20 to 50 per cent in-hospital mortality [1,2]. The recommended
treatment includes vasoactive drugs as Terlipressin (triglycyl lysine vasopressin) as adjuvant to
endoscopic variceal band ligation (EVBL) [1-3]. Terlipressin has a different bioavailability profile
from vasopressin, enabling it to be given as intermittent intravenous injections, with no effect on
plasminogen activator and it is believed to have an intrinsic vasoconstrictor effect. In clinical trials
it showed to induce a lower incidence of severe adverse reactions [4,5].
It has also been suggested that the use of terlipressin combined with intravenous albumin
infusion may improve renal function in patients with cirrhosis and type 1 hepatorenal syndrome
[6,7] (HRS). In spite of its benefits, adverse effects related to its vasoconstrictive properties have been
reported. Although rare, terlipressin–related ischemic adverse effects acute ST-segment elevation
myocardial infarction [8], severe lactic acidosis [9], extremities ischemia and gangrene [10] had
been reported. We report two patients with severe lower limb skin necrosis not reversible even after
terlipressin withdrawal.
Case Presentation
Case 1
A 47 year old man was admitted in the ICU with upper digestive bleeding due to gastric varices.
He had been submitted to a Warren procedure sixteen years before for the treatment of portal
hypertension due to hepatosplenic mansonic schistosomiasis. Terlipressin 1 mg every 4 hours was
administered and an upper digestive endoscopy with cianoacrilate injection was performed. 24
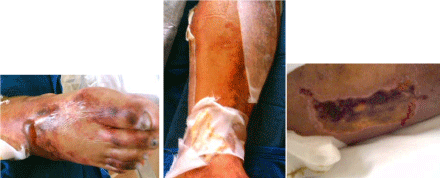
hours later the patient developed skin blisters on the legs with progression to severe skin necrosis
even after terlipressin suspension (Figure 1). Resection of the necrotic area and a skin graft were
performed. The patient presented a good outcome and was discharged from hospital on the 48th day.
Case 2
A 68 year old man was admitted in the ICU with liver insufficiency with ascites, hepatic
encephalopathy and hepatorenal syndrome after a right extended hepatectomy for colorectal
metastasis. Terlipressin 1 mg every 6 hours and albumin were prescribed. 48 hrs later the patient
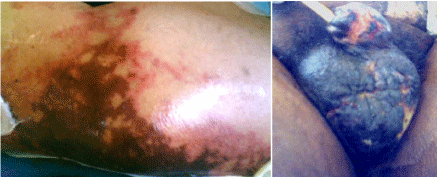
presented severe skin necrosis of the legs and scrotum irreversible even after terlipressin suspension
(Figure 2). The patient died due to multiple organ failure on the 38th postoperative day.
Figure 1
Figure 2
Discussion
Terlipressin is a prohormone (lysine-vasopressin) that after intravenous administration is cleaved by endothelial peptidases, allowing prolonged release of vasopressin. Vasopressin is a potent
splanchnic vasoconstrictor but its use was abandoned many years ago in most countries because
of its severe vascular side effects. Terlipressin, a vasopressin analogue, has similar effects reducing hepatic venous pressure gradient, variceal pressure and azygos blood flow and, due to its hemodynamic effects, have been used in the therapy of variceal hemorrhage [11,12].
Despite its excellent vasopressive effects, vasopressin analogues may potentially impair macro-hemodynamics, oxygen transport and microvascular blood flow. Terlipressin can induce ischemic complications, especially in patients with severe hypovolemic shock [13], and it is not indicated in patients with cardiovascular disease (arterial disease with severe obstruction, cardiac insufficiency, arrhythmias, and hypertension).
A meta-analysis showed efficacy and safety with the use of terlipressin in the treatment of hepatorenal syndrome [14]. The most frequent adverse effects reported were abdominal pain, arrhytmias and skin lesions, all of them reversible after dose reduction. Recently, cases reported describe ischemic complications of various parts of the body,such as hips, abdominal region, trunk, legs and scrotal region [15-19]. Nevertheless, the complications were reversible after terlipressin suspension. The terlipressin dosage administered to our patients was those recommended on the world’s literature. Our patients presented irreversible skin lesions even after terlipressin suspension.
We believe that due to its bioavailability profile, terlipressin is slowly cleaved by endothelial peptidases to vasopressin [20], being possible that its vasoconstrictive action persists even after its interruption and that these prolonged action combined with a possible direct vasoconstrictive property could be responsible for the severe skin necrosis observed in our patients. Recent studies have suggested that terlipressin may also be administered as a low-dose continuous infusion (1.3-2.6 μg /kg/) in the early course of distributive shock [21] with near the same hemodynamic effects [22,23].
Our data emphasizes the need of randomized trials for the establishment of the optimal terlipressin dosage, administration regimen and the occurrence of unwanted side-effects.
References
- Seo YS, Park SY, Kim MY, Kim JH, Park JY, Yim HJ, et al. Lack of difference among terlipressin, somatostatin, and octreotide in the control of acute gastroesophageal variceal hemorrhage. Hepatology. 2014; 60: 954-963.
- D'Amico G, De Franchis R, Cooperative Study Group. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology. 2003; 38: 599-612.
- Soderlund C, Magnusson I, Torngren S, Lundell L. Terlipressin (triglycyl-lysine vasopressin) controls acute bleeding oesophageal varices. A double-blind, randomized, placebo-controlled trial. Scand J Gastroenterol 1990; 25: 622-630.
- Burroughs AK. Pharmacological treatment of acute variceal bleeding. Digestion. 1998; 59: 28-36.
- Dib N, Oberti F, Calès P. Current management of the complications of portal hypertension: variceal bleeding and ascites. CMAJ. 2006; 174: 1433-1443.
- Boyer TD, Sanyal AJ, Wong F, Frederick RT, Lake JR, O'Leary JG, et al. Terlipressin plus albumin is more effective than albumin alone in improving renal function in patients with cirrhosis and Hepatorenal Syndrome type 1. Gastroenterology. 2016. 150: 1579-1589.
- Gerbes AL, Gulberg V. Progress in treatment of massive ascites and hepatorenal syndrome. World J Gastroenterol. 2006; 12: 516-519.
- Lee MY, Chu CS, Lee KT, Lee HC, Su HM, Cheng KH, et al. Terlipressin-related acute myocardial infarction: a case report and literature review. Kaohsiung J Med Sci. 2004; 20: 604-608.
- Foresti V, Parisio E, Ungaro A, De Filippi G, Confalonieri F. Terlipressin-induced metabolic acidosis. Recenti Prog Med. 1991; 82: 240-241.
- Lee JS, Lee HS, Jung SW, Han WS, Kim MJ, Lee SW, et al. [A case of peripheral ischemic complication after terlipressin therapy]. Korean J Gastroenterol. 2006; 47: 454-457.
- Saner F, Kavuk I, Lang H, Biglarnia R, Frühauf NR, Schäfers RF, et al. Terlipressin and gelafundin: safe therapy of hepatorenal syndrome. Eur J Med Res. 2004; 9: 78-82.
- Moreau R, Durand F, Poynard T, Duhamel C, Cervoni JP, Ichaï P, et al. Terlipressin in patients with cirrhosis and type 1 hepatorenal syndrome: a retrospective multicenter study. Gastroenterology. 2002; 122: 923-930.
- Feu F, Ruiz del Arbol L, Bañares R, Planas R, Bosch J. Double-blind randomized controlled trial comparing terlipressin and somatostatin for acute variceal hemorrhage. Variceal Bleeding Study Group. Gastroenterology. 1996; 111: 1291-1299.
- Fabrizi F, Dixit V, Martin P. Meta-analysis: terlipressin therapy for the hepatorenal syndrome. Aliment Pharmacol Ther. 2006; 24: 935-944.
- Oh JE, Ha JS, Cho DH, Yu GJ, Shim SG. [A case of ischemic skin necrosis after glypressin therapy in liver cirrhosis]. Korean J Gastroenterol. 2008; 51: 381-384.
- Vaccaro F, Giorgi A, Riggio O, De Santis A, Laviano A, Rossi-Fanelli F. Is spontaneous bacterial peritonitis an inducer of vasopressin analogue side-effects? A case report. Dig Liver Dis. 2003; 35: 503-506.
- Mégarbané H, Barete S, Khosrotehrani K, Izzedine H, Moguelet P, Chosidow O, et al. Two observations raising questions about risk factors of cutaneous necrosis induced by terlipressin (Glypressin). Dermatology. 2009; 218: 334-337.
- Taşliyurt T, Kutlutürk F, Erdemır F, Yelken BM, Yilmaz A, Kisacik B, et al. Ischemic skin necrosis following terlipressin therapy: report of two cases and review of the literature. Turk J Gastroenterol. 2012; 23: 788-791.
- Ozel Coskun BD, Karaman A, Gorkem H, BuÄŸday I, PoyrazoÄŸlu OK, Senel F. Terlipressin-induced ischemic skin necrosis: a rare association. Am J Case Rep. 2014; 15: 476-479.
- Burroughs AK. Pharmacological treatment of acute variceal bleeding. Digestion. 1998; 2: 28-36.
- Morelli A, Ertmer C, Lange M, Westphal M. Continuous terlipressin infusion in patients with septic shock: less may be best, and the earlier the better?. Intensive Care Med. 2007; 33: 1669-1670.
- Umgelter A, Reindl W, Schmid RM, Huber W. Continuous terlipressin infusion in patients with persistent septic shock and cirrhosis of the liver. Intensive Care Med. 2008; 34: 390-391.
- Cavallin M, Piano S, Romano A, Fasolato S, Frigo AC, Benetti G, et al. Terlipressin given by continuous intravenous infusion versus intravenous boluses in the treatment of hepatorenal syndrome: A randomized controlled study. Hepatology. 2016; 63: 983-992.