Case Report
Systemic Sarcoidosis First Manifesting in a Tattoo in the Setting of Immune Checkpoint Inhibition: A Case Report
Kim C1, Gao J2, Shannon V3, Mays S4 and Radtke SA2*
1Department of Genetics, The University of Texas MD Anderson Cancer Center, USA
2Department of Genitourinary Medical Oncology, The University of Texas MD Anderson Cancer Center, USA
3Department of Pulmonary Medicine, The University of Texas MD Anderson Cancer Center, USA
4Department of Dermatology, The University of Texas Health Science Center at Houston McGovern Medical
School, USA
*Corresponding author: Arlene Siefker-Radtke, Department of Genitourinary Medical Oncology, The University of Texas MD Anderson Cancer Center, 1155 Hermann Pressler Dr, Houston, TX, 77030, USA
Published: 02 Jul, 2016
Cite this article as: Kim C, Gao J, Shannon V, Mays S,
Radtke SA. Systemic Sarcoidosis First
Manifesting in a Tattoo in the Setting of
Immune Checkpoint Inhibition: A Case
Report. Ann Clin Case Rep. 2016; 1:
1032.
Abstract
A 52-year-old man with metastatic urothelial carcinoma of the left renal pelvis was treated with
surgical resection and chemotherapy. Immune checkpoint therapy with anti-CTLA-4 (ipilimumab)
and anti-PD1 (nivolumab) was initiated after the patient failed conventional chemotherapy. The
patient had several large cosmetic tattoos placed on both arms many years prior to the cancer
diagnosis. While on immunotherapy, thickened, hyperkeratotic papular lesions developed along the
outer edges of the tattoos. Similar lesions also appeared over his face, and he developed arthralgias.
Surveillance imaging following two cycles of immunotherapy demonstrated a mixed response
of the lung metastases but definite progression of disease in the surgical bed and enlargement of
mediastinal, hilar and retroperitoneal lymph nodes. Immunotherapy was discontinued after the
second cycle due to presumed disease progression. A biopsy of one of the skin lesions revealed
noncaseating granulomatosis, consistent with cutaneous sarcoidosis. The skin and mediastinal
lymph node biopsies were culture-negative. Pathology of the lymph nodes also demonstrated
noncaseating granulomas with no evidence of malignancy, indicating that the patient’s radiographic
finding “mediastinal disease progression” was actually Lofgren syndrome sarcoidosis. After several
weeks of high dose prednisone therapy, significant regression of the diffuse lymphadenopathy
and skin lesions was seen on CT imaging studies, while the true metastatic lesions within the lung
parenchyma and surgical bed remained unchanged.
This case demonstrates that extrapulmonary sarcoidosis associated with immune checkpoint
blockade can be mistaken for malignancy and may be misleading, resulting in premature
termination of potentially efficacious treatments. Therefore, when in doubt, it is important to
conduct histopathology of metastases before discontinuation of immune checkpoint therapy.
Keywords: Immune checkpoint blockade; Urothelial cancer; Sarcoid
Abbreviations
irAE: Immune-Related Adverse Events; CLTA-4: Cytotoxic T-Lymphocyte Antigen-4; PD-1: Programmed Cell Death Protein 1; CT: Computerized Tomography; ESR: Erythrocyte Sedimentation Rate
Introduction
The premise of immune checkpoint blockade is to overcome the negative regulatory
mechanisms that suppress T cell activation [1,2]. Initially, antitumor T cell activation requires
two interactions: first between the T-cell receptor and tumor antigen and then between CD28 on
the T cell and B7 proteins on antigen-presenting cells. However, the resulting T cell response is
modulated by inhibitory factors that prevent unchecked immune activation. For instance, cytotoxic
T-lymphocyte antigen-4 (CTLA-4), binds to the B7 proteins, attenuating complete T cell activation
[3]. Ipilimumab, an anti-CTLA-4 monoclonal antibody, prevents this attenuation and allows T cells
to mount immune responses to tumor antigens [4]. Similarly, the programmed cell death protein 1
(PD-1) dampens T cell activation by binding to the PD-L1 ligand, resulting in apoptosis in T cells.
Nivolumab, an anti-PD-1 antibody, hinders this immune checkpoint [5,6]. Since ipilimumab and
nivolumab block different immune checkpoints, they are increasingly implemented in a combination
regimen to further promote tumor regression [7,8]. Both drugs are novel and advantageous because
they do not require identification of specific tumor antigens: rather, they “release the brakes” [9] on the body’s endogenous immune antitumor response.
Ipilimumab and nivolumab both display side effects, termed immune-related adverse events (irAEs) [10,11]. The most common known irAEs are skin-related (dermatitis/rash), gastrointestinal (enterocolitis/diarrhea), endocrine, and hepatic. Each irAE has a broad range of severity in terms of recorded clinical observations [10,11]. Immune checkpoint inhibiting agents represent a relatively new class of drugs Rare irAEs must be properly noted in order to properly address the diversity of adverse events across patient cases.
Here, we present a case of sarcoidosis, which arose from a tattoo in a patient with metastatic urothelial cancer while on therapy with ipilimumab and nivolumab. Although most cases of skin sarcoid remain limited to the skin, our patient not only developed progressive skin sarcoid, but also experienced full-blown Lofgren syndrome with erythema nodosum, arthralgias in his finger joints and knees, and biopsy-proven sarcoidosis in his mediastinal lymph nodes. Previous findings have reported pulmonary sarcoidosis following immune checkpoint blockade, but not in multi-organ, multi-symptomatic contexts of systemic syndromes like disseminated Lofgren’s [12,13]. This case demonstrates that immune checkpoint therapy can cause exacerbation of sarcoidosis, which can confound treatment options by masquerading as malignancy.
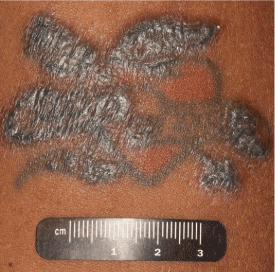
Figure 1
Figure 1
Sarcoid reaction manifesting in a tattoo after immune checkpoint blockade
Thickened, hyperkeratotic papular lesions developed along the outer edges of the tattoo after the patient received ipilimumab and nivolumab combination immune checkpoint blockade therapy. Although the patient did not want the tattoo to be biopsied, histopathology of a similar nodular lesion on his left naris revealed sarcoid.
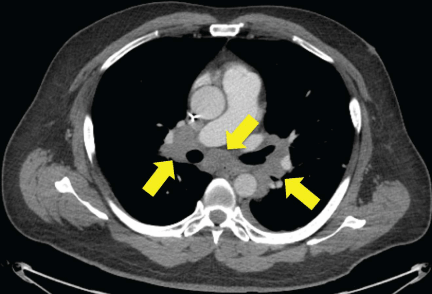
Figure 2
Figure 2
CT of hilar lymphadenopathy, which was initially believed to be tumor progression.
After three doses of immunotherapy, restaging CT scans showed interval enlargement in the known tumor mass in the bed of kidney resection and new hilar lymphadenopathy. Immunotherapy was discontinued due to disease progression on CT scans, and he began chemotherapy with gemcitabine and cyclophosphamide. However, a hilar lymph node biopsy via bronchoscopy
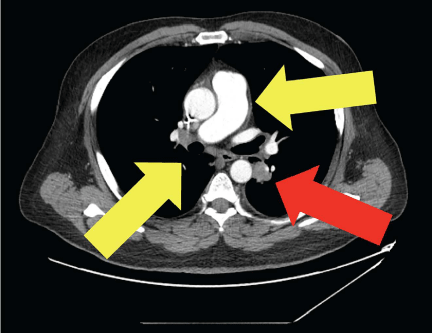
Figure 3
Figure 3
Methylprednisolone treatment decreased sarcoid lymphadenopathy
Methylprednisolone sodium succinate administration resolved the skin lesions, arthralgias, and tattoo changes of the patient’s Lofgren’s syndrome. Repeat CT revealed a decrease in mediastinal and hilar lymphadenopathy (yellow arrows), suggesting a clinical response of sarcoidosis to the steroids. However, the lymphadenopathy due to malignance (red arrow) persisted.
Case Presentation
A 52-year-old man with metastatic urothelial carcinoma of the left renal pelvis was enrolled in a clinical trial with immune checkpoint therapy. He had failed prior chemotherapy with gemcitabine, paclitaxel and doxorubicin, as well as with methotrexate, vinblastine, doxorubicin, and cisplatin. Approximately 60 days after beginning treatment with a combination of nivolumab (3 mg/kg intravenously (i.v.)) and ipilimumab (1 mg/kg i.v.), he noted papules and thickening along the black ink of two of his tattoos (Figure 1). He also developed progressively enlarging papules around his eyes and nares. After three doses of immunotherapy, his restaging computerized tomography (CT) scans showed interval enlargement in the known tumor mass in the bed of kidney resection and new hilar lymphadenopathy (Figure 2). Immunotherapy was discontinued due to disease progression on CT scans, and he began chemotherapy with gemcitabine and cyclophosphamide. In the meantime, he was referred to dermatology for evaluation of changes in his facial skin and tattoos.
The dermatologist performed a biopsy of a papule adjacent to his left naris, which was consistent with sarcoidosis. Special stains were negative for fungal, bacterial, and acid-fast organisms. Immunohistochemistry was negative for spirochetes. Treatment with hydroxychloroquine (plaquenil) 200 mg by mouth twice a day was initiated for sarcoidosis. After starting plaquenil, the patient reported progressive skin papules, which increased in size and number despite discontinuation of immunotherapy. Systemic bloodwork was negative for hypercalcemia, elevated angiotensin-converting enzyme, and elevated 1,25-dihydroxyvitamin D, which are common serum markers of sarcoidosis. However, erythrocyte sedimentation rate (ESR) was elevated at 70 mm/hr and 90 mm/hr (normal: <20 mm/hr) on two separate occasions. An ophthalmologic exam indicated that there was no uveitis or sarcoid involvement of the eyes.
The patient also experienced further induration of the black ink in his tattoos as well as tenderness and swelling of the joints of his fingers and knees. In addition, he was noted to have tender nodules anterior to his shins, bilaterally consistent with erythema nodosum. A hilar lymph node biopsy via bronchoscopy showed no evidence of cancer but was consistent with sarcoid. The diagnosis of Lofgren’s syndrome was made based on the triad of hilar lymphadenopathy, arthralgias, and erythema nodosum.
In light of the rapid progression of the sarcoid after discontinuation of immunotherapy, the patient was started on methylprednisolone sodium succinate (Solu-Medrol) 1 mg/kg i.v. twice a day. The skin lesions, arthralgias, and tattoo changes resolved, and the patient was subsequently tapered off the steroids. Repeat CT scans (Figure 3) with contrast of the chest demonstrated an increase in metastatic lesions involving the lungs and liver capsule, but a decrease in mediastinal and hilar lymphadenopathy, suggesting a clinical response of the sarcoidosis to steroids.
Discussion
Sarcoidosis has been reported in patients receiving immune checkpoint therapy, and has also been reported as arising from tattoos. This is the first case of sarcoid in a tattoo that occurred during use of immune checkpoint therapy. Overall, immune checkpoint therapy has transformed the field of cancer care due to its unique ability to harness the body’s antitumor immune response and yield long-lasting efficacy. Removing the cellular processes that inhibit antitumor T cell activity, has led to durable clinical responses in melanoma and genitourinary malignancies [7,14,15]. However, as demonstrated in this case, immune activation may have nonspecific systemic consequences. This is especially true in disorders of immune etiology, such as sarcoidosis, where excessive Th1 activity induces release of cytokines such as IL-2 and IFN-γ [16]. Cutaneous and pulmonary sarcoidosis have been previously documented as drug reactions to ipilimumab, establishing a putative link between immune checkpoint blockade and the induction of sarcoidosis [17,18]. Moreover, the clinical presentation of our patient reveals that the combination of ipilimumab/nivolumab can induce systemic extrapulmonary sarcoidosis in the form of Lofgren’s syndrome.
From a clinical perspective, it is critical to differentiate between malignant progression and immunotherapy-related sarcoidosis. We note that extrapulmonary sarcoidosis caused by immune checkpoint blockade can be mistaken for malignancy, although, in this case, the patient had known tumor progression in the bed of kidney resection as well. As a result, we emphasize the importance of conducting histopathological analyses when evaluating visceral metastases in the context of immunotherapy. Sarcoidosis is a manageable side effect and should not dictate the course of cancer treatment.
Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the editor of this journal.
Acknowledgements
CK is funded by the National Center for Advancing Translational Sciences of the National Institutes of Health (under Award Numbers TL1TR000369 and UL1TR000371) and the American Legion Auxiliary (ALA). Written consent was obtained from the patient for publication of the case report.
Author’s Contributions
CK drafted the manuscript. All other authors contributed to editing the manuscript. VS, SM, and ASR treated the patient.
References
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012; 12: 252-264.
- Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol. 2015; 33: 1974-1982.
- Allison JP, Krummel MF. The Yin and Yang of T cell costimulation. Science. 1995; 270: 932-933.
- Peggs KS, Quezada SA, Korman AJ, Allison JP. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr Opin Immunol. 2006; 18: 206-213.
- Reiss KA, Forde PM, Brahmer JR. Harnessing the power of the immune system via blockade of PD-1 and PD-L1: a promising new anticancer strategy. Immunotherapy. 2014; 6: 459-475.
- McDermott DF, Atkins MB. PD-1 as a potential target in cancer therapy. Cancer Med. 2013; 2: 662-673.
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013; 369: 122-133.
- Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015; 372: 2006-2017.
- Weintraub K. Drug development: Releasing the brakes. Nature. 2013; 504: S6-8.
- Yoho R, Rivera JJ, Renschler R, Vardaxis VG, Dikis J. A biomechanical analysis of the effects of low-Dye taping on arch deformation during gait. Foot (Edinb). 2012; 22: 283-286.
- Gao J, He Q, Subudhi S, Aparicio A, Zurita-Saavedra A, Lee DH, et al. Review of immune-related adverse events in prostate cancer patients treated with ipilimumab: MD Anderson experience. Oncogene. 2015; 34: 5411-5417.
- Gangadhar TC, Vonderheide RH. Mitigating the toxic effects of anticancer immunotherapy. Nat Rev Clin Oncol. 2014; 11: 91-99.
- Berthod G, Lazor R, Letovanec I, Romano E, Noirez L, Mazza Stalder J, et al. Pulmonary sarcoid-like granulomatosis induced by ipilimumab. J Clin Oncol. 2012; 30: e156-159.
- Eckert A, Schoeffler A, Dalle S, Phan A, Kiakouama L, Thomas L. Anti-CTLA4 monoclonal antibody induced sarcoidosis in a metastatic melanoma patient. Dermatology (Basel). 2009; 218: 69–70.
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010; 363: 711-723.
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015; 348: 56-61.
- Chen ES, Moller DR. Etiologies of Sarcoidosis. Clin Rev Allergy Immunol. 2015; 49: 6-18.
- Reule RB, North JP. Cutaneous and pulmonary sarcoidosis-like reaction associated with ipilimumab. J Am Acad Dermatol. 2013; 69: e272-273.