Case Report
Case Report of Granulocytic Sarcoma in Bilateral Temporal Bones
Gao Z1, Chi Fang-lu1* and Wang Shu-yi2
1Department of Otorhinolaryngology, Fudan University, China
2Department of Pathology, Fudan University, China
*Corresponding author: Fang-lu Chi, Department of Otorhinolaryngology, Fudan University, No.83, Fenyang Road, Shanghai 200031, China
Published: 27 Jun, 2016
Cite this article as: Gao Z, Chi Fang-lu, Wang Shu-yi.
Case Report of Granulocytic Sarcoma
in Bilateral Temporal Bones. Ann Clin
Case Rep. 2016; 1: 1024.
Abstract
Objective: We report an extremely rare case of granulocytic sarcoma in bilateral temporal bones.
Methodology: Case report and a review of related literature.
Results: The patient was initially performed neoplasm ectomy then systemic chemotherapy in
his left ears. After surgery the patient got a significant improvement of his left hearing. One year
later systemic chemotherapy was established again after a biopsy of his right ear. Nowadays he is
still under the treatment of acute myeloid leukemia without recurrence of temporal granulocytic
sarcoma.
Conclusion: Granulocytic sarcoma involving temporal bones is rare. Its diagnosis depends on
immunohistochemistry. Systemic chemotherapy is the first therapeutic choice. Surgical application
should be limited to biopsy and symptomatic relief.
Keywords: Granulocytic sarcoma; Temporal bone; Conductive hearing loss
Introduction
Granulocytic sarcoma (GS) is a localized extramedullary tumor composed of immature myeloid cells. It's an uncommon disease and often described in association with acute myeloid leukemia (AML). In head and neck, it usually involves the skull, gingiva, orbit, facial skinand even paranasal sinus [1-4]. Though rare, GS can affect temporal bone and produce symptoms which areeasily confused with other diseases. Here we report a case of GS in bilateraltemporal bones.
Case Presentation
In November 2006, a 36-year-old Chinese man, with a history of AML (subtype M3) which was
in remission, presented to our hospital for otalgia and hearing loss in bilateral ears for a month's
duration. Previously in December 2000, he was diagnosed as AML (M3) and received chemotherapy;
in January 2001 he achieved complete remission, after that he remained in continuous complete
remission for 5 years. He denied the history of facial palsy, hearing loss or vertigo.
On admission, otoscopy showed his left external acoustic meatus was completely blocked by a
soft-tissue mass. His right external acoustic meatus was stenotic because of swelling of its posterior
and anterior portions, only a small part of the right tympanic membrane could be seen, which seemed
normal. No pathological enlargement of lymph nodes or organomegaly was noticed. Laboratory
studies including complete blood counts and serum biochemistry showed normal values. CT scan
of both temporal bones revealed both external acoustic meatus, especially the left, were filled with
masses. No bony lesions were found (Figure 1). The CT scan was also suggestive of right mastoiditis
(Figure 1). Pure-tone audiogram revealed conductive hearing loss in bilateral ears with air-bone
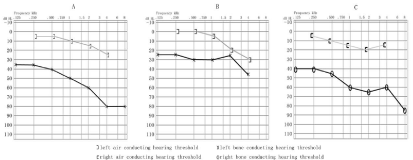
gaps about 35 to 45 dB in the right and30 to 55 dB in the left (Figure 2).
Because of the patient's history of AML, he was first sent to another hospital to review his
leukemia. In that admission, his bone marrow aspirate didn't show any relapse of AML. Though
GS couldn't be excluded, other diseases, such as sarcoma, were considered preferentially. Few days
later, the patient was submitted to surgery in our hospital. Aneoplasm ectomy of the left external
acoustic meatus was performed though a retroauricular approach. During the surgery, a tumor
was seen originating from the walls of external acoustic meatus, involving part of the tympanic
membrane. The tympanic cavity was normal and the ossicular chain was intact. The mass and
the tympanic membrane were removed, the ear canal was widened and the temporalis fascia
was harvested to rebuild the tympanic membrane. The specimen
was performed HE and immunohistochemistry staining. In HE
staining, the lesion was consistent showing pleomorphic cells
having blastic and hyperchromatic nucleus with large cytoplasm. In
immunohistochemistry, it was positive for myeloperoxidase, LCA,
lysozyme, CD43, and CD117 (Figure 3), but negative for CD20 and
CD7.These results lead to the conclusive diagnosis of GS.
Because of the diagnosis of GS, the patient immediately received
a systemic chemotherapy. But due to his poor compliance, only one
course of the treatment was finished. One month after the operation,
the patient got a complete recovery from his left ear symptoms, and
pure-tone audiogram showed a significant increase of air conductive
hearing (Figure 2). However, the symptoms of his right ear hardly
changed and the otalgia became even severe. In December 2007, the
patient received operation of his right ear in our hospital. A lesion
similar with the left was seen in the canal, the lesion was completely
removed and diagnosed as GS by pathology. After surgery, systemic
chemotherapy began. Three months later, the patient recovered from
right otalgia, however the hearing hardly improved. Nowadays, the
patient is still under the treatment of AML, and clinical examination
or CT scans haven't detected any signs of temporal GS recurrence.
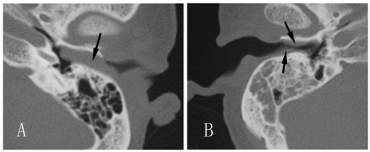
Figure 1
Figure 1
(A) Axial CT scan of the left ear obtained before treatment shows
a complete blockage of the external acoustic meatus by a soft tissue mass
(arrow), without bone destructions. (B) Axial CT scan of the right ear obtained
before treatment shows mass (arrow) in external acoustic meatus causing a
stenosis, it also revealed an asymptomatic mastoiditis.
Figure 2
Figure 2
Pure-tone audiogram obtained before the surgery of both ear (A, C) and after the surgery of left ear (B).
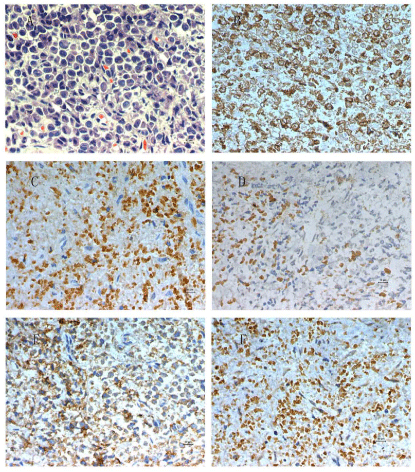
Figure 3
Figure 3
Histology of the surgical specimen from the left ear.
(A) Diffuse infiltration of large mononuclear cells with wide cytoplasm and polymorphic nuclei. Immunohistochemical staining is positive for (B) lysozyme, (C) CD
43 antigen, (D) CD117 antigen, (E) LCA antigen and (F) myeloperoxidase. The bars in photos equal to 10 μm.
Discussion
GS, also termed myeloid sarcoma or "chloroma", is a rare disease.
GS has a close relationship with AML. As reported before, about 2–8
% of patients with AML develop GS [5]. It may present at any time
of the course of AML [6]. Though all subtypes of AML could develop
GS, the French-American-British (FAB) subtypes M2, M4, M5 are
most frequently observed [7]. Certain chromosome abnormalities are associated with a higher incidence of GS, particularly t (8; 21), and
less frequently inv (16) (p13;q22) [8]. GS can also arise in patients
with other types of myelo proliferative disorders, such aschronic
myelogenous leukemia blastic transformation and myelodysplastic
syndrome [9-11].
GS in temporal bone is quite rare. Chapman and Johnson
described the first one in 1980 [12]. Temporal GS is most commonly
found in patients below the age 30, and its incidence has no deviation
in gender or side. GS could span the whole temporal bone, but
mastoid is most often involved. Presenting symptoms of temporal GS
include facial palsy, otalgia, tinnitus and conductive hearing loss. To
our knowledge, GS usually affects unilateral temporal bone; the case
presented here is the first one with bilateral temporal GS.
Recognition of GS is important for timely treatment. As long as
a patient present for neoplasm with a history of AML, GS should be
considered. Some investigations can provide clues for diagnosis. On
CT scans, GS may appear as a well-defined enhancing mass sometimes
with osteolytic destruction. On MRI, GS may be homogeneously
hyper intense on fat saturated T2-weighted images, hypo intense
on T1-weighted images and moderate enhancing after intravenous
gadolinium contrast administration [13]. However, it's impossible to
confirm GS from imaging findings alone. Making a conclusion often
needs biopsy for histological diagnosis.
The differential diagnosis of temporal GS comprises external
canal cholesteatoma, temporal lymphoma and temporal sarcoma.
Temporal GS can be easily parted from external canal cholesteatoma
for its lack of keratinized debris. However, it's difficult to distinguish
GS from lymphoma and sarcoma by routine investigations. At that
condition, immunohistochemical analysis should be performed.
The most sensitive markers for GS include CD3, CD43 and
myeloperoxidase [14], others such as CD20, LCA and lysozyme can
also be used. In addition, the tumor should be negative for B and T
cell markers [15]
.
The optimal treatment of GS is not clear since there is not enough
data and large prospective studies in the literature. Nowadays GS is
mostly suggested to treat by systemic chemotherapy with or without
radiotherapy [16]. Other therapeutic choices include hematopoietic
stem cell transplantation and targeted therapy. Surgery alone is not
recommended for the high incidence of AML and extramedullary
relapse [17]. In this case, we used surgery combining systemic
chemotherapy to treat our patient. In our opinion, Because of the
anatomic complexity of temporal bone, biopsy in it most frequently
needs a surgical approach. Surgery should also be reserved for patients
having conductive hearing loss or facial palsy, for its rapid effect of
symptomatic relief. To our patient, the surgery not only contributed
to biopsy, but also successfully improved his left hearing.
Conclusion
GS involving bilateral temporal bones is extremely rare. It should be considered for patients present for neoplasm with a history of acute myeloid leukemia. Conclusive diagnosis of granulocytic sarcoma needs immunohistochemical analysis. Systemic chemotherapy is the first therapeutic choice. Surgical application should be limited to biopsy and symptomatic relief.
References
- Suzer T, Colakoglu N, Cirak B, Keskin A, Coskun E, Tahta K. Intracerebellar granulocytic sarcoma complicating acute myelogenous leukemia: a case report and review of the literature. J Clin Neurosci. 2004; 11: 914-917.
- Matsushita K, Abe T, Takeda Y, Takashima H, Takada A, Ogawa Y, et al. Granulocytic sarcoma of the gingiva: two case reports. Quintessence Int. 2007; 38: 817-820.
- Kumar J, Seith A, Bakhshi S, Kumar R, Kumar A, Sen S. Isolated granulocytic sarcoma of the orbit. Eur J Haematol. 2007; 78: 456.
- Prades JM, Alaani A, Mosnier JF, Dumollard JM, Martin C. Granulocytic sarcoma of the nasal cavity. Rhinology. 2002; 40: 159-161.
- Liu PI, Ishimaru T, McGregor DH, Okada H, Steer A. Autopsy study of granulocytic sarcoma (chloroma) in patients with myelogenous leukemia, Hiroshima-Nagasaki 1949–1969. Cancer. 1973; 31: 948-955.
- Balleari E, Panarello S, Capello E, Grosso M, Passalia C, Pitto P, et al. Granulocytic sarcoma: an unusual cause of spinal cord compression. Int J Clin Oncol. 2007; 12: 234-237.
- Porto L, Kieslich M, Schwabe D, Zanella FE, Lanfermann H. Granulocytic sarcoma in children. Neuroradiology. 2004; 46: 374-377.
- Sevinc A, Buyukberber S, Camci C, Koruk M, Savas MC, Turk HM, et al. Granulocytic Sarcoma of the Colon and Leukemic Infiltration of the Liver in a Patient Presenting with Hematochezia and Jaundice. Digestion. 2004; 69: 262-265.
- Hamadani M, Tfayli A, Sethi S, Awab A, Hamdani N. Granulocytic Sarcoma Manifesting as Multiple Skeletal Lesions. Am J Med Sci. 2005; 330: 139-143.
- Campidelli C, Agostinelli C, Stitson R, Pileri SA. Myeloid sarcoma: extramedullary manifestation of myeloid disorders. Am J Clin Pathol. 2009; 132: 426-437.
- Yilmaz AF, Saydam G, Sahin F, Baran Y. Granulocytic sarcoma: a systematic review. Am J Blood Res. 2013; 3: 265-270.
- Chapman P, Johnson SA. Mastoid chloroma as relapse in acute myeloid leukaemia. J Laryngol Otol. 1980; 94: 1423-1427.
- Graham A, Hodgson T, Jacubowski J, Norfolk D, Smith C. MRI of perineural extramedullary granulocytic sarcoma. Neuroradiology. 2001; 43: 492-495.
- Dettrick AJ, Robertson T, Morris KL. Diagnosis of granulocytic sarcoma on pleural effusion cytology: report of a case. Diagn Cytopathol. 2004; 31: 126-128.
- Menasce LP, Banerjee SS, Beckett E, Harris M. Extra-medullary myeloid tumour (granulocytic sarcoma) is often misdiagnosed: a study of 26 cases. Histopathology. 1999; 34: 391-398.
- Tsimberidou AM, Kantarjian HM, Estey E, Cortes JE, Verstovsek S, Faderl S, et al. Outcome in patients with nonleukemic granulocytic sarcoma treated with chemotherapy with or without radiotherapy. Leukemia. 2003; 17: 1100-1103.
- Wilson CS, Medeiros LJ. Extramedullary Manifestations of Myeloid Neoplasms. Am J Clin Pathol. 2015; 144: 219-239.