Case Report
Antro-Pyloric Stenosis and Ectopic Pancreas Carcinoma: What is This?
De Simone B1*, Catena F1 and Charre L2
1Department of Trauma and Emergency Surgery, University Hospital of Parma, Italy
2Centre Hospitalier Renée Dubos, Service de Chirurgie Digestive et Epatobiliare, France
*Corresponding author: De Simone Belinda, University Hospital of Parma, Via Gramsci 15, 43100 Parma, Italy
Published: 05 Jun, 2016
Cite this article as: De Simone B, Catena F, Charre L.
Antro-Pyloric Stenosis and Ectopic
Pancreas Carcinoma: What is This?.
Ann Clin Case Rep. 2016; 1: 1019.
Abstract
Ectopic pancreas (EcP) is an uncommon congenital anomaly that can be found anywhere along the Gastro-Intestinal tract, most frequently in the stomach (antrum), in the duodenum or in the jejunum. EcP is often asymptomatic and benign, but it can show the same pathological features of the pancreas gland, even malignant transformation. Preoperative diagnosis is difficult. The prognosis of adenocarcinoma developed from ectopic pancreas is still unknown. We report the clinical case of a female 67 years old patient, admitted in our department for vomiting. The aim of our case report with review of the literature is to highlight that in clinical practice EcP is a rare pathological condition but it has to be considered in the differential diagnosis of upper gastrointestinal tract disease, because of the risk of malignant degeneration.
Keywords: Ectopic pancreas; Submucosal gastric cancer; Wedge resection; Laparoscopy; Endoscopy
Introduction
Ectopic pancreas (EcP) is an uncommon congenital anomaly defined as pancreatic tissue
abnormally situated, without connection to the normal pancreas but provided with its own vascular
and ductal system, as a result of fetal migration of pancreatic tissue during gastrointestinal formation
with further development in ectopic area [1-3]. The first case report of heterotopic pancreas was
presented in 1729 by Schultz [1,3,4] and the first histopathological description was made in 1859
by Klob [1-3]; it has been found in 0.6-13% of autopsies [1-5] and most of the patients affected are
asymptomatic.
EcP in the upper GastroIntestinal tract (GI) was located frequently in the duodenum (25-35%),
gastric antrum (25-60%, relatively frequently along the greater curvature and posterior and anterior
walls, whereas it rarely occurs along the lesser curvature) and jejunum; rarely it can be detected
in the esophagus, lung, mesentery, spleen, gallbladder, biliary tract and Meckel's diverticulum [6].
Von Heinrich proposed a histological classification of heterotopic pancreas in 1909, modified
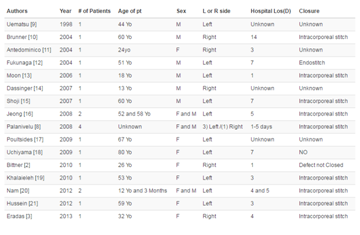
by Gaspar Fuentes in 1973, as summarized in the (Table 1) [7]. EcP tissue can show the same
pathological features of the pancreas gland, even malignant transformation. Generally the patient
affected is asymptomatic or he presents with aspecific gastrointestinal symptoms as vomiting,
abdominal pain and dyspepsia; when the location is in the stomach, the evolution of the lesion
can be the ulceration, the bleeding or it can enlarge till the stenosis, above all in prepyloric region.
Most of time, the lesion is detected incidentally. The correct preoperative diagnosis is not common;
abdominal ultrasound (US) and computed tomography (CT) are often useless to do differential
diagnosis with the other upper gastrointestinal malignancies. On CT, EcP appears as a well defined
oval or round mass with smooth or serrated margins in the gastric antral or intestinal wall or as
a mesenteric mass similar to a gastrointestinal stromal tumor or a carcinoid tumor [7,8]. In the
majority of cases, pancreatic tissue in the stomach spreads only in the submucosa and endoscopic
examinations, such as upper endoscopy (UE) and endoscopic ultrasound (EUS), allow the use of
targeted fine needle aspiration biopsy, necessary for the diagnosis, even if the result of cytology is
often inconclusive (in about 50% of cases) [9]. Definitive diagnosis is made on the histo-patological
examination of the surgical specimen. Gastric adenocarcinoma developing from ectopic pancreatic
tissue is a rare condition: only an hundred cases are reported in literature, and the prognosis is
unknown [2,10-12]. We report the clinical case of a female 67 years old patient submitted to gastric
resection for indeterminate pyloric stenosis.
Table 1
Case Report
A 63-year-old woman was admitted in our department of general
surgery for vomiting and dyspeptic symptoms. One year before, the
patient had undergone laparoscopic proctocolectomy with coloanal
anastomosis (colonic J-pouch reconstruction), and temporary
ileostomy, for infiltrative, well differentiated, rectal adenocarcinoma,
classified "pT3N1". At the admission, her vital parameters were
normal. At the physical examination, no abdominal pain or palpable
masses were found. Laboratory investigations were normal, except for
gamma glutamyl transferasis at 58 U/L (normal < 38); normal value
of neoplastic marker CEA and CA 19-9 at 110 UI/L (normal value
< at 35). The patient, before being hospitalized, in the context of the
follow-up program after colorectal surgery for cancer, had undergone
abdominal US examination that showed a discrete dilatation of
descending duodenum (D2) with hypo-echoic lesion associated.
Consequently the patient was submitted to abdominal Computed
Tomography (CT) that showed an aspecific wall thickening of the
antro-pyloric region without lymphadenopathy. The UE revealed a
pre-pyloric stenosis, such as an extrinsic compression, in the absence
of mucosal lesions. Endoscopic biopsies of gastric mucosa were made
and showed a pattern of chronic gastritis of the antrum, associated
with Helicobacter Pylori infection, without intestinal metaplasia and/
or glandular atrophy. After multidisciplinary discussion of the case,
on the basis of these inconclusive results and considering the clinical
features suspicious for primary or metastatic neoplastic disease, we
decided to perform an explorative laparoscopy in agreement with
the radiologists, the oncologists and the gastroenterologists of our
hospital.
The surgical procedure, because of the presence of multiple
lymphadenopathies, was converted in explorative laparotomy. The
exploration of the abdomen showed a neoplastic lesion of 3.5 cm,
localized at the pyloric region, associated with sub-centimeters
lymphadenopathies of the pancreatic region, of the duodenum,
of the greater curvature and of the hepatic pedicle, without signs
of peritoneal carcinomatosis. We decided to perform a partial
gastrectomy according to Finsterer's technique with gastro-jejunal
anastomosis, cholecystectomy, retroportal and duodenopancreatic
lymphoadenectomy. No surgical complications occurred in the early
postoperative period and the patient was discharged on day 10 after the
intervention, with adequate pain relievers. One month after surgery,
the wound healed completely, no infectious complications and no
signs of neoplastic disease's relapse was found. Definitive histological
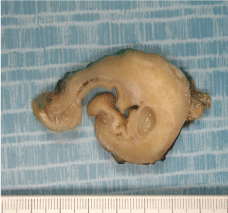
examination of the gastric lesion, (Figure 1), showed the presence of
submucosal antrum-pyloric tumor, combining heterotopic, normal
and dysplastic, glands, and carcinomatous infiltrative glands, such
as adenocarcinoma developed from ectopic pancreas, classified
Heinrich type 3 The immunohistochemistry was positive for CK7,
CK19, CK 20 and MUC2, negative to MUC1. No lymph node
metastases were found. The patient didn't receive chemotherapy. A
three-month follow-up was decided for the first 3 years, then every
six months during the following 2 years, with a physical examination
of the patient, the serum dosage of the neoplastic markers CEA and
CA 19-9; the patient will undergo abdominal CT alternated to hepatic
US every 3 months during the first 3 years and then every 6 months,
for the following 2 years. Every year, for 5 years, the patient will be
submitted to chest CT. At the last examination, 1 year after surgery,
the patients had no signs of recurrent disease.
Figure 1
Discussion
Malignant transformation in gastric EcP has been reported in
several cases, an hundred in literature. Some scientists argue that,
being the risk of malignant degeneration of the ectopic pancreatic
tissue, it should be removed as soon as possible; others suggest the
conservative management, until the patient is asymptomatic; no
consensus on the timing of the follow up, both for the conservative
management and after surgery [2-6]. The risk is that when the patient
begins to show aspecific gastrointestinal symptoms, he could be in
advanced neoplastic disease. Armstrong et al. found a correlation
between the presence of GI symptoms and the size of the lesion
(greater than 1.5 cm), and the extent of mucosal involvement [5,7-9].
Preoperative diagnosis of EcP is difficult with US and CT [8,9]. It is
located predominantly in the submucosa. UE and EUS examination
are the first investigation tools used to evaluate submucosal lesions in
the upper gastrointestinal tract. The endoscopic picture of EcP in the
stomach has been described as an elevated delomorphic submucosal
tumor that has a normal mucosa over it with characteristic central
umbilication, but this endoscopic picture is not always present, as in
our patient [9-12].
According to the endosonographic features, Haas et al.
classified the EcP into 2 types: a separated type and a fusion type,
as summarized in the (Table 2). Park et al. [13] modified Hase's
classification, creating a new classification as summarized in the (Table 3), to facilitate preoperative diagnosis and differentiation
from GIST [14]. Our patient didn't have EUS. Surgical resection is
the best therapeutic option. Our patient was submitted to partial
gastrectomy for suspected primary neoplasm or metastatic lesion of
the gastric antrum. In literature, EcP can be resected by traditional
laparotomy with a wedge resection, by laparoscopic sleeve resection
or by endoscopic submucosal dissection [9]. Minimally invasive
techniques are associated with two potential problems: laparoscopy
may be unable to determine the location of gastric small mucosal
tumors because of their small size or intraluminal growth pattern;
besides complications as stenosis or damage of cardia or pylorus
could occur when the lesions are near to these anatomical structures [15-18]. Harold et al. reported 4 cases of EcP in the stomach
that underwent transgastric laparoscopic resection without early
postoperative complications. Lee et al. [16] reported a case of
robotic-assisted laparoscopic wedge resection for EcP in a 14 years
old girl with good outcomes; he concluded that laparoscopy assisted
Billroth I gastrectomy is a less invasive surgical technique with less
surgical trauma, less impaired nutrition, less pain, rapid recovery of
gastrointestinal function, shorter hospital stay, without decrease in
operative curability compared with conventional open gastrectomy.
Kang et al. [17] developed a technique called laparoscopic endoscopic
cooperative surgery (LECS): the endoscopic assistant cuts the exact
edges from the gastric lumen; the following laparoscopic tumor
resection is aided by endoscopy, to reduce complications and to
have direct intraluminal visualization confirming that the tumor has
been totally removed, that there is no bleeding from the sutures lines
and that there are no perforation. This hybrid surgical technique is
indicated for gastric small mucosal tumors, polyps with low potential
for malignancy and early stage localized gastric carcinomas [17].
In his retrospective study conducted on 101 patients consecutively
submitted to partial, proximal or distal gastrectomy using LECS,
Kang reported that intraoperative bleeding was limited and recovery
of bowel function was rapid with low postoperative morbidity and
no postoperative mortality. Excision of EcP has to be R0 in all cases
because of unknown prognosis. The biology of adenocarcinoma
developed on ectopic pancreas is unknown but no lymph node
metastases are described in literature [6].
Patients submitted to surgery with adenocarcinoma on EcP,
need a long term follow up. In the literature, only ten cases have been
reported, with a survival ranging between six months and ten years,
all with a life expectancy longer than in case of orthotopic pancreas
adenocarcinoma [6,19-22].
Table 2
Table 3
Conclusion
Malignant transformation of ectopic pancreatic tissue is described in literature [6,7]. Preoperative diagnosis is difficult; US, CT, endoscopic findings and biopsy are often inconclusive. Diagnosis is made on histological examination. Surgical treatment is the best therapeutic option: complete surgical resection should be performed. Laparoscopic wedge resection and endoscopic dissection are safe and reproducible surgical techniques as classic laparotomy, also for larger tumors, with good outcomes in specialized centers. The prognosis of adenocarcinoma arising from ectopic pancreas is still unknown. Long term follow up after surgery is needed.
References
- Habibi H, Devuni D, Rossi L. Ectopic pancreas: a rare cause of abdominal pain. Conn Med. 2014; 78: 479-480.
- . Lemaire J, Delaunoit T, Molle G. Adenocarcinoma arising in gastric heterotopic pancreas. Case report and review of the literature. Acta Chir Belg. 2014; 114: 79-81.
- Buczek T, Puzdrowski W, Lenekowski R, Kruszewski WJ. [Ectopic pancreas imitating gastric neoplasm -- a case report]. Przegl Lek. 2013; 70: 761-763.
- Zawada I, Lewosiuk A, Hnatyszyn K, Patalan M, Woyke S, Kostyrka R, et al. [Ectopic pancreas mimicking advanced gastric malignancy--case report]. Pol Merkur Lekarski. 2012; 32: 246-249.
- Esquivel C, Ballario F, García S, Giraudo P, Esteban Granero L. [Submucosal gastric tumour: heterotopic pancreas. A case report and review of the literature]. Acta Gastroenterol Latinoam. 2011; 41: 234-237.
- OOkamoto H, Kawaoi A, Ogawara T, Fujii H. Invasive ductal carcinoma arising from an ectopic pancreas in the gastric wall: a long-term survival case. Case Rep Oncol. 2012; 5: 69-73.
- Okasha HH, Al-Bassiouni F, El-Ela MA, Al-Gemeie EH, Ezzat R. A retroperitoneal neuroendocrine tumor in ectopic pancreatic tissue. Endosc Ultrasound. 2013; 2: 168-170.
- Kim JY, Lee JM, Kim KW, Park HS, Choi JY, Kim SH, et al. Ectopic pancreas: CT findings with emphasis on differentiation from small gastrointestinal stromal tumor and leiomyoma. Radiology. 2009; 252: 92- 100.
- Makarewicz W, Bobowicz M, Dubowik M, Kosinski A, Jastrzebski T, Jaskiewicz J. Endoscopic submucosal dissection of gastric ectopic pancreas. Wideochir Inne Tech Maloinwazyjne. 2013; 8: 249-252.
- Makni A, Rebai W, Azzouz H, Daghfous A, Ayadi S, Ben Safta Z. Ectopic pancreas mimicking submucosal gastric tumor. Acta Gastroenterol Belg. 2011; 74: 483-484.
- Fukumori D, Matsuhisa T, Taguchi K, Minato M. Ectopic gastric pancreatic cancer: report of a case. Hepatogastroenterology. 2011; 58: 740-744.
- Barbe L, Levy P, Bougaran J, Just J, Mal F, Ruszniewski P, et al. [Cystic and mucinous lesion in an antral ectopic pancreas]. Gastroenterol Clin Biol. 1998; 22: 824-826.
- Park SH, Kim GH, Park do Y, Shin NR, Cheong JH, Moon JY, et al. Endosonographic findings of gastric ectopic pancreas: a single center experience. J Gastroenterol Hepatol. 2011; 26: 1441-1446.
- Matsushita M, Takakuwa H, Nishio A. Endosonographic features of gastric adenomyoma, a type of ectopic pancreas. Endoscopy. 2003; 35: 621-622.
- Catalano F, Rodella L, Lombardo F, Silano M, Tomezzoli A, Fuini A, et al. Endoscopic submucosal dissection in the treatment of gastric submucosal tumors: results from a retrospective cohort study. Gastric Cancer. 2013; 16: 563-570.
- Lee YT, Lin H, Guo JC, Yan SL, Hou HJ, Lai YS, et al. Laparoscopy-assisted billroth I gastrectomy for ectopic pancreas in the prepyloric region. Case Rep Gastroenterol. 2012; 6: 712-719.
- Kang WM, Yu JC, Ma ZQ, Zhao ZR, Meng QB, Ye X. Laparoscopicendoscopic cooperative surgery for gastric submucosal tumors. World J Gastroenterol. 2013; 19: 5720-5726.
- Galatioto C, Goletti O, Franceschi M, Buccianti P, Neri E, Armillotta N, et al. Laparoendoscopic treatment of gastric ectopic pancreas. Surg Laparosc Endosc Percutan Tech. 1999; 9: 160-164.
- Goto S, Okazaki T, Koga H, Miyano G, Arakawa A, Yao T, et al. Ectopic pancreas presenting as a submucosal gastric tumor: case report and literature review. Pediatr Surg Int. 2011; 27: 107-109.
- Emerson L, Layfield LJ, Rohr LR, Dayton MT. Adenocarcinoma arising in association with gastric heterotopic pancreas: A case report and review of the literature. J Surg Oncol. 2004; 87: 53-57.
- Jeong HY, Yang HW, Seo SW, Seong JK, Na BK, Lee BS, et al. Adenocarcinoma arising from an ectopic pancreas in the stomach. Endoscopy. 2002; 34: 1014-1017.
- Barbe L, Levy P, Bougaran J, Just J, Mal F, Ruszniewski P, et al. [Cystic and mucinous lesion in an antral ectopic pancreas]. Gastroenterol Clin Biol. 1998; 22: 824-826.