Case Report
The Use of Continuous Glucose Monitoring for Sport in Type 1 Diabetes
Esther O'Sullivan1,2,*, Abdulrahman A1, Manhas J1, Linnane H2, Gurney M2 and Fitzgerald C1
*Corresponding author: Esther O'Sullivan, Department of School of Medicine, National University of Ireland Galway, University Road, Galway, Ireland
Published: 20 Jun, 2018
Cite this article as: O'Sullivan E, Abdulrahman A, Manhas
J, Linnane H, Gurney M, Fitzgerald
C. The Use of Continuous Glucose
Monitoring for Sport in Type 1 Diabetes.
Ann Clin Case Rep. 2018; 3: 1523.
Abstract
The benefits of exercise for patients with Type 1 Diabetes (T1D) are difficult to balance with associated glycaemic excursions. The aim of this study was to show that Continuous Glucose Monitoring (CGM) could reduce glycaemic excursions in patients with T1D already using insulin pumps, exercising at moderate to high intensity. Questionnaires were used to identify T1D patients using insulin pumps and naïve to CGM use, who reported regular exercise. 6 were enrolled and trained on Enlite sensor use with Medtronic Minimed Paradigm® Veo™ system, and given activity trackers and written advice on adjustment of insulin or carbohydrate intake for exercise. Resting heart rate (HR) and age were used to determine HR surrogates of moderate and high intensity exercise. They were to exercise as usual for 3 weeks (run-in week, week 1 and week 2), using the activity trackers and heart rate monitors. PAID, HFSII, DTQ and Gold scores were completed prior to run-in and at the end. The downloaded sensor glucose data was used to compare the change in time in range (glucose 3.9-10.0 mmol/l) from week 1 to week 2 For the duration of exercise this time in range glucose Range increased from 72 ± 20 to 88 ± 16%, p=0.05. The time in hypoglycaemia range (glucose < 3.9 mmol/l) went from 3.9 ± 7.9 to 2.4 ± 4.8%, p=0.39. The time in hyperglycaemia range (>10 mmol/l) reduced from 24 ± 19 to 10 ± 17%, p=0.04. These results demonstrate the benefit of CGM use for patients with T1DM doing moderate to high intensity exercise.
Introduction
Exercise is recommended to patients with Type 1 Diabetes to improve their cardiovascular health and reduce their risk factors for cardiovascular disease [1]. However exercise, especially moderate to high intensity exercise, can lead to excursions in glucose levels that are difficult to manage [2]. Education about appropriate insulin dose adjustments for different types of exercise can be very helpful, but is not always enough to eliminate this problem [3]. The use of Insulin pump therapy instead of multiple daily injections can also help [4] but even patients using this form of insulin delivery, where the insulin basal rate can be adjusted readily, often continue to struggle to maintain good glycaemic control around the time of exercise [5,6]. Nocturnal hypoglycaemia after aerobic exercise sessions is a typical problem [7,8], but hypoglycaemia and hyperglycaemia during or immediately after exercise also occur [9]. Hyperglycaemia is more likely to occur after high intensity or anaerobic exercise [10]. Patients sometimes target hyperglycaemia before exercise as a mechanism of avoiding hypoglycaemia during it. A useful tactic to avoid hypoglycaemia during exercise is to intersperse a 10 second sprint into a lower intensity aerobic exercise session [11]. Studies in controlled settings have demonstrated the benefit of using real-time Continuous Glucose Monitoring (CGM) to inform insulin and carbohydrate adjustments to avoid wide glycaemic excursions [12,13]. The aim of this study was to examine the benefit of CGM for patients involved in regular moderate to high intensity exercise, in the real-life setting.
Methods
Approval for the study was obtained from Galway University Hospital Clinical Research Ethics
committee. Questionnaires were sent to all type 1 Diabetes patients using insulin pumps attending
the Diabetes clinic at Galway University Hospital. Those naïve to real-time CGM use, who reported
regular (at least twice per week) moderate to high intensity exercise were invited to participate. 6
were enrolled and trained on Enlite sensor use with Medtronic Minimed Paradigm® Veo™ system.
All were given written general advice on adjustment of insulin or carbohydrate intake, for different
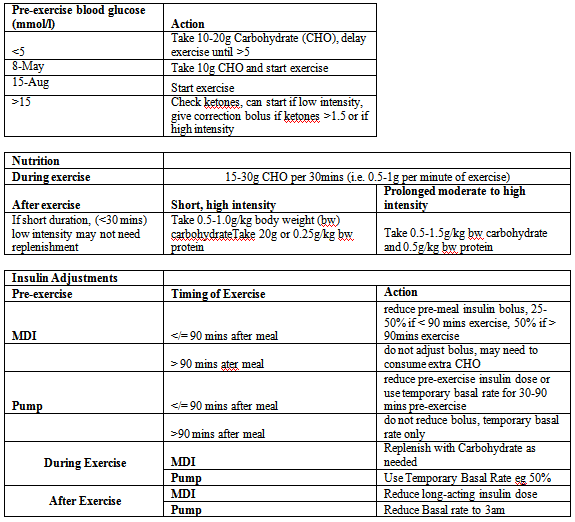
exercise intensities, duration and proximity to mealtime (Figure 1). This is adapted from Exercise
management in type 1 diabetes: a consensus statement [14].
They were also given Polar ProTrainer 5™ watches and shown how
to use them with the accompanying heart rate monitor chest strap, to
monitor their heart rate during exercise sessions. We measured their
resting heart rate, and together with age this was used to calculate
the age predicted maximum heart rate (220-age=HRmax). Using the
Karvonen method we determined heart rate reserve (HRR=HRmax-
HRrest). HR ranges 40% to 70% and 70% to 80% of HRR added
to HRrest were used as surrogates of moderate and high intensity
exercise respectively. Weight, BMI and BP were recorded at baseline,
and a blood sample was sent to test Hba1c (IFCC assay). They were
instructed to exercise as usual for 3 weeks, turning the Polar watch
on for the duration of exercise. The Moves app was installed on their
own smartphones that they were asked to carry always to monitor
their level of activity at times when they were not exercising. At the
end the run-in (week-1) subjects were contacted to troubleshoot any
calibration or sensor change problems. If they consistently wore the
sensor, exercised at least twice per week and used the Polar™ watch
for exercise during the run-in week they were included in the study.
2 subjects were not included. In one case this was because regular
exercise was not carried out due to an injury, in the other case it was
because there was difficulty using Polar watch and heart rate monitor
for exercise sessions which involved rugby training.
Problem areas in Diabetes (PAID) [15], Hypoglycaemia fear
survey II (HFSII) [16], Diabetes Technology Questionnaire (DTQ)
[17] were completed prior to run-in and at the end of the study and the
Gold score [18] was obtained from each individual before the study
to assess their level of hypoglycaemia unawareness. (This is a simple
score from 1-7 in response to the question: Do you know when your
hypos are commencing? Where 1 is where you are always aware and
7 means you are never aware). These are all validated questionnaires
designed to quantify quality of life and hypoglycaemia unawareness
and changes in these parameters over time.
The downloaded sensor glucose data on Carelink™ Personal
(Medtronic software for patient and health professional review of
insulin and glucose data) was analysed in SPSS. Thresholds for glucose
levels were set as follows: target range 3.9 mmol/l to 10 mmol/l,
hypoglycaemia range <3.9 mmol/l, hyperglycaemia range >10 mmol/l.
All available sensor glucose values were included in the analysis (one
value is provided every 5 minutes while the sensors are worn). The
periods of time the patients spent exercising were determined from
the heart rate monitor-where glucose data associated with a heart rate at least 0.4 × (HRR-HR rest) was included. Student’s paired t-test was
used to compare time in the range, in week 1 to week 2.
Additional recommendations
If first-time exercise there is a prolonged hypoglycaemia risk so
the basal rate should be reduced for the entire night using temporary
basal rate setting, if on MDI the long acting dose should be reduced
that day/night. Hypoglycaemia risk is also higher if exercise is carried
out on sequential days, or there was a hypoglycaemic event on the day
preceding exercise. Alcohol also increases the risk of hypoglycaemia.
If hyperglycaemia is encountered post-exercise (especially likely
if mod/high intensity exercise at the anaerobic/lactate threshold) a
cool-down over 20 minutes to 30 minutes will reduce the need to give
a correction bolus. A 10-second sprint done before and/or during
low/moderate intensity exercise will elevate glucose levels and reduce
the risk of hypoglycaemia during or after exercise.
Figure 1
Figure 2a
Figure 2a
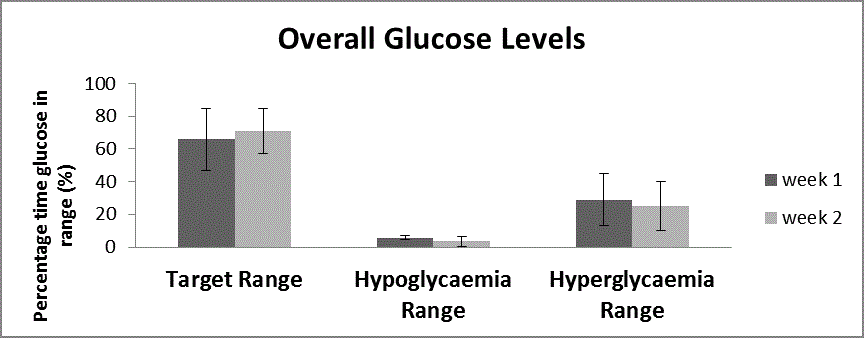
All available glucose values over the 2 weeks for the study are divided into the 3 different ranges and week 1 is compared to week 2.
Figure 2b
Figure 2b
The glucose values that correspond to the time of exercise (determined from the Polar watch HR data (all time that HR + HRR > 40% HRmax) was
divided into the 3 target ranges and compared between week 1 and week 2.
Figure 2c
Results/h2>
Figure 2 the sensor glucose values were binned into time in target,
time in hypoglycaemia range and time in hyperglycaemia range and
are shown as percentage in each range. Figure 2a all available glucose
values over the 2 weeks for the study are divided into the 3 different
ranges and week 1 is compared to week 2. Figure 2b the glucose values
that correspond to the time of exercise (determined from the Polar
watch HR data (all time that HR + HRR > 40% HRmax) was divided
into the 3 target ranges and compared between week 1 and week 2.
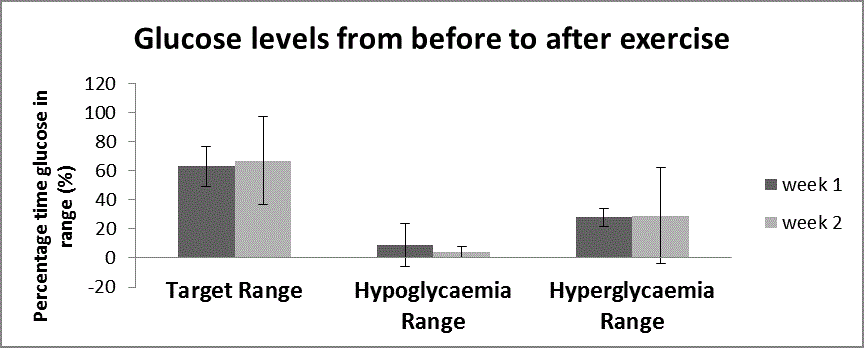
Figure 2c shows the glucose values from 1 hour before to 4 hours after
exercise. 3 of the subjects wore sensors for all 21 days of the study, 1
wore it for 19 days. The percentage of missing data points did not differ
significantly from week to week and duplicates were removed. There
was an increase in the time in the target glucose range during exercise,
in week 2 compared to week 1 72.4 ± 20% to 88.1 ± 15.6% p=0.05),
and a reduction in the time in the hyperglycaemia range (24 ± 19%
to 10 ± 17% p=0.04 (Figure 2b). There was no significant reduction
in hypoglycaemia, however the improvement in the time in target is
not all attributable to reduction in the time in the hyperglycaemia
range-the mean time in the hypoglycaemia during exercise reduced
from 3.9 ± 7.9% to 2.4 ± 4.8%, p=0.39. When the sensor glucose data
for the entire 2 week period was evaluated, there was no significant
difference from week 1 to week 2 (Figure 2a), or for the time from 1
hour before to 4 hours after exercise (Figure 2c).
The Gold score ranged from 1-4 indicating no hypoglycaemia
unawareness in one individual, and a mild to moderate degree in the
other subjects. The other quality of life measures showed low levels
of anxiety (HFS II Worry scale scores were 13 ± 16 out of a possible
0-72), and behaviour due to fear of hypoglycaemia (14 ± 7 out of a
possible 0-48). The PAID scores were also moderate (16 ± 14 out of
a possible 0-80). Nonetheless all of the QOL scores showed a nonsignificant
trend toward improving following use of the CGM for the
duration of the study.
Table 1
Discussion
This small real-life study demonstrates the potential benefit
of using CGM for exercise. We showed that there was a significant
improvement in the time that interstitial glucose was in the target range (3.9 mmol/l to 10 mmol/l) during exercise. These patients were
involved in a variety of types of exercise, cycling, running, soccer and
weight lifting and all had improvements in their glycaemic control
during exercise between week 1 and week 2 of the study. Although
there was no significant change in the time in the hypoglycaemia
range from week 1 to week 2, there was a trend in this direction, and
the time spent in the hypoglycaemia range was small. This difference
didn’t reach significance probably in part due to the small number
of subjects. The same reason may explain the lack of difference in
the time in target range overall from week 1 to week 2 (including
time exercising and not), where time in the hypoglycaemia reduced
from 5.6 ± 3.1% to 3.4 ± 1.2% p=0.15, and hyperglycaemia time
reduced from 29 ± 16% to 25 ± 15% p=0.11. Similar results were
found for the time from 1 hour before and exercise session started
to 4 hours afterward. This is a period that carries a high risk for wide
glycaemic excursions; we expect a larger study would show significant
reductions in time in the hypoglycaemia and hyperglycaemia ranges
during this period. Our study showed a high proportion of time in
hypoglycaemia during this time (8.9 ± 14.9% in week 1, 4.1 ± 3.8%
in week 2).
The subjects used the CGM system for a run-in week and there
were no overall significant improvements demonstrated between that
week and subsequent weeks, suggesting that these patients needed
some time to get used to the device and how to adjust their insulin
according to the CGM glucose data available. The only input from
the Diabetes research team was at the beginning of the study – when
subjects were given basic instructions on insulin adjustment for
exercise (Table 1), and shown how to use the study devices. There
was a further contact after the run-in week, in person or by phone,
to trouble-shoot any problems, but we did not review their data
or provide individualised advice until the end of the study. Each
subject had access to Carelink™ Personal and reviewed their data
both in realtime and retrospectively to help to inform insulin dose
adjustment. All of the patients were already insulin pump users, so
the benefits they gained demonstrate the role of CGM in addition to
insulin pump therapy in the setting of exercise. HbA1cs varied from
on target to significantly elevated indicating a wide range of glycaemic
control at baseline.
The HFSII and PAID questionnaires show there was a moderate
level of fear of hypoglycaemia and anxiety around management of their
Diabetes in general. The DTQ showed the most consistent responses
at the end of the study were of a slight or great reduction in the stress
around participating in sport and avoiding hypoglycaemia(Results
not shown). All 4 found the CGM device user-friendly, for the 2
patients that did not pass the run-in phase CGM compliance was not
the issue, it was due to inability to exercise and difficulty with the
heart rate monitor and exercise watch.
Technology for the management of Diabetes continues to advance
and health care professionals involved in Diabetes management are
challenged with the question of which patients are likely to benefit
from the use of CGM. As it stands CGM in the UK and Ireland is
funded in cases where there is a specific need such as hypoglycaemia
unawareness or recurrent hypoglycaemia. More research is needed to
determine what other patients are likely to benefit not just in terms
of HbA1c, but also in terms of reduction of glycaemic excursions and
improvement in quality of life measures. There is some evidence to
suggest the amplitude of glycaemic excursions and not just HbA1c
is correlated and positively associated with development of Diabetes
complications, but more longitudinal CGM based studies are needed
[19]. CGM use has the potential to reduce these excursions thereby
possibly reduce the risk of development or progression of these
complications. There is some evidence to demonstrate the benefits
to be gained in terms of quality of life for patients who access CGM
after previously relying on finger-stick tests for self-monitoring of
blood glucose [20]. Not all patients report reduced anxiety, but the
continuous glucose data along with trend arrows, predictive alarms,
and low-glucose suspend features in some cases provide great
reassurance and in cases of hypoglycaemia unawareness have the
potential to avoid life threatening hypoglycaemia. There are tools that
help predict whether individual children with T1D will benefit from
the use of CGM particularly in terms of consistent use [21]. Further
study is needed to determine how to predict the type of adults with
T1D that will benefit.
To date cost-effectiveness analyses have not shown clear
indications for the use of these devices [22]. The cost involved is not
just that of the sensor enabled pump and sensors, but also the cost
of the Diabetes educator hours involved in training patients on how
to use these devices effectively. Here we have shown that a variety
of insulin pump users can achieve benefits from the additional use
of CGM with minimal input form the Diabetes team. All of these
patients had previously attended a structured education course so
had good baseline knowledge of how to adjust their insulin doses
themselves. There also may be an element of selection bias and the
individuals who agreed to participate may represent a cohort with
a high level of motivation. However we feel this study demonstrates
that a short trial of CGM use can quickly determine whether patients
already using insulin pump therapy, are likely to be compliant with
CGM use. Those patients participating in regular moderate to high
intensity exercise are a cohort who can quickly benefit from the
addition of CGM to improve their glucose time in the target range,
and also improve their quality of life.
Type 1 Diabetes has a major impact on an individual’s lifestyle,
including their ability to partake in exercise safely and enjoyably.
Progress in technology for the treatment of Diabetes makes this more
attainable. CGM whether as an integrated sensor augmented pump
system or stand-alone device is available for selected patients, but
many more stand to benefit from it especially those involved in regular
moderate to high intensity exercise. New developments hold even
more promise to improve the lives of these individuals. One study
in the real-life setting to investigate the use of CGM along with an
accelerometer and algorithm that incorporated the exercise intensity,
showed reduction in hypoglycaemia [23]. The future incorporation
of heart rate and other data into a closed loop device should further
facilitate avoidance of glycaemic excursions around exercise. In
the meantime, as we have shown in this small pilot study, sensor
enabled insulin pumps have the potential to allow these patients to
exercise while reducing their glycaemic excursions and anxiety about
hypoglycaemia.
Table 2
Compliance with Ethical Standards
Conflict of interest: none. All procedures performed in studies involving human participants were in accordance with the ethical standards of GUH and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Acknowledgement
Dr Amir Shafat Department of Physiology NUI,G loaned the Polar ProTrainer 5™ watches for this study. Catriona Fitzgerald was the recipient of a Summer Student Bursary from the Irish Endocrine Society.
References
- Yardley JE, Hay J, Abou-Setta AM, Marks SD, McGavock J, et al. A systematic review and meta-analysis of exercise interventions in adults with type 1 diabetes. Diabetes Res Clin Pract. 2014; 106: 393-400.
- García-García F, Kumareswaran K, Hovorka R, Hernando ME, et al. Quantifying the acute changes in glucose with exercise in type 1 diabetes: a systematic review and meta-analysis. Sports Med. 2015; 45: 587-599.
- Rabasa-Lhoret R, Bourque J, Ducros F, Chiasson JL. Guidelines for premeal insulin dose reduction for postprandial exercise of different intensities and durations in type 1 diabetic subjects treated intensively with a basal-bolus insulin regimen (ultralente-lispro). Diabetes Care. 2001. 24: 625-630.
- Yardley JE, Iscoe KE, Sigal RJ, Kenny GP, Perkins BA, Riddell MC. Insulin pump therapy is associated with less post-exercise hyperglycemia than multiple daily injections: an observational study of physically active type 1 diabetes patients. Diabetes Technol Ther. 2013; 15: 84-88.
- Zaharieva DP, MC Riddell. Prevention of exercise-associated dysglycemia: a case study-based approach. Diabetes Spectr. 2015; 28: 55-62.
- Franc S, Daoudi A, Pochat A, Petit MH, Randazzo C, Petit C, Duclos M, et al, Insulin-based strategies to prevent hypoglycaemia during and after exercise in adult patients with type 1 diabetes on pump therapy: the DIABRASPORT randomized study. Diabetes Obes Metab. 2015; 17: 1150-1157.
- Matthew D Campbell, Mark Walker, Richard M Bracken, Daniel Turner, Emma J Stevenson, Javier T Gonzalez, et al. Insulin therapy and dietary adjustments to normalize glycemia and prevent nocturnal hypoglycemia after evening exercise in type 1 diabetes: a randomized controlled trial. BMJ Open Diabetes Res Care. 2015; 3: e000085.
- MacDonald MJ. Postexercise late-onset hypoglycemia in insulin-dependent diabetic patients. Diabetes Care. 1987; 10: 584-588.
- Campbell MD, Walker M, Trenell MI, Luzio S, Dunseath G, Tuner D, et al. Metabolic implications when employing heavy pre- and post-exercise rapid-acting insulin reductions to prevent hypoglycaemia in type 1 diabetes patients: a randomised clinical trial. PLoS One. 2014; 9: e97143.
- Shetty VB, Fournier PA, Davey RJ, Retterath AJ, Paramalingam N, Roby HC, et al. Effect of Exercise Intensity on Glucose Requirements to Maintain Euglycemia During Exercise in Type 1 Diabetes. J Clin Endocrinol Metab. 2016; 101: 972-980.
- Bussau VA, Ferreira LD, Jones TW, Fournier PA. et al. The 10-s maximal sprint: a novel approach to counter an exercise-mediated fall in glycemia in individuals with type 1 diabetes. Diabetes Care. 2006; 29: 601-606.
- Riddell MC, J Milliken. Preventing exercise-induced hypoglycemia in type 1 diabetes using real-time continuous glucose monitoring and a new carbohydrate intake algorithm: an observational field study. Diabetes Technol Ther. 2011; 13: 819-825.
- Garg S, Brazg RL, Bailey TS, Buckingham BA, Slover RH, Klonoff DC, et al. Reduction in duration of hypoglycemia by automatic suspension of insulin delivery: the in-clinic ASPIRE study. Diabetes Technol Ther. 2012; 14: 205-209.
- Riddell MC, Gallen IW, Smart CE, Taplin CE, Adolfsson P, Lumb AN, et al. Exercise management in type 1 diabetes: a consensus statement. Lancet Diabetes Endocrinol. 2017; 5: 377-390.
- Polonsky WH, Anderson BJ, Lohrer PA, Welch G, Jacobson AM, Aponte JE, et al. Assessment of diabetes-related distress. Diabetes Care. 1995; 18: 754-760.
- Gonder-Frederick LA, Schmidt KM, Vajda KA, Greear ML, Singh H, Shepard JA, et al. Psychometric properties of the hypoglycemia fear survey-ii for adults with type 1 diabetes. Diabetes Care. 2011; 34: 801-806.
- Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Validation of measures of satisfaction with and impact of continuous and conventional glucose monitoring. Diabetes Technol Ther. 2010; 12: 679-684.
- Høi-Hansen T, Pedersen-Bjergaard U, Thorsteinsson B. Classification of hypoglycemia awareness in people with type 1 diabetes in clinical practice. J Diabetes Complications. 2010; 24: 392-397.
- Smith-Palmer J, Brändle M, Trevisan R, Orsini Federici M, Liabat S, Valentine W. Assessment of the association between glycemic variability and diabetes-related complications in type 1 and type 2 diabetes. Diabetes Res Clin Pract. 2014; 105: 273-284.
- Kubiak T, Mann CG, Barnard KC, Heinemann L. Psychosocial Aspects of Continuous Glucose Monitoring: Connecting to the Patients' Experience. J Diabetes Sci Technol. 2016; 10: 859-863.
- Neylon OM, Skinner TC, O'Connell MA, Cameron FJ. A novel tool to predict youth who will show recommended usage of diabetes technologies. Pediatr Diabetes. 2016; 17: 174-183.
- Riemsma R, Corro Ramos I, Birnie R, Büyükkaramikli N, Armstrong N, Ryder S, et al. Integrated sensor-augmented pump therapy systems [the MiniMed(R) Paradigm Veo system and the Vibe and G4(R) PLATINUM CGM (continuous glucose monitoring) system] for managing blood glucose levels in type 1 diabetes: a systematic review and economic evaluation. Health Technol Assess. 2016; 20: 1-251.
- Stenerson M, Cameron F, Payne SR, Payne SL, Ly TT, Wilson DM, et al. The impact of accelerometer use in exercise-associated hypoglycemia prevention in type 1 diabetes. J Diabetes Sci Technol. 2015; 9: 80-85.