Case Report
Good Outcome Following Catastrophic Cerebral Sinus Thrombosis due to Heparin-Induced Thrombocytopenia: Case Report and Review of Literature
Anudariya Dean1, Stephanie Zyck2, Grahame Gould3, Elena Schmidt4 and Julius Gene Latorre5*
1College of Medicine, SUNY Upstate Medical University Hospital, USA
2Department of Neurosurgery, SUNY Upstate Medical University Hospital, USA
3Department of Neurosurgery, SUNY Upstate Medical University Hospital, USA
4Department of Neurology and Neurosurgery, SUNY Upstate Medical University Hospital, USA
5Department of Neurology and Neurosurgery, SUNY Upstate Medical University Hospital, USA
*Corresponding author: Julius Gene Latorre, Department of Neurology, SUNY Upstate Medical University Hospital, 750 East Adams Street, Syracuse, NY 13210, USA
Published: 31 May, 2018
Cite this article as: Dean A, Zyck S, Gould G, Schmidt
E, Latorre JG. Good Outcome
Following Catastrophic Cerebral Sinus
Thrombosis due to Heparin-Induced
Thrombocytopenia: Case Report and
Review of Literature. Ann Clin Case
Rep. 2018; 3: 1517.
Abstract
Introduction: Systemic anticoagulation with heparin is the primary treatment of acute cerebral
venous sinus thrombosis (CVT). Treatment of CVT due to heparin-induced thrombocytopenic
thrombosis (HITT) is a management conundrum. In the acute phase when continuous parenteral
anticoagulant is necessary, argatroban, a direct thrombin inhibitor, has been used for HITassociated
thrombosis. In cases of catastrophic CVT with severe neurologic compromise or when
anticoagulation does not result in clinical improvement, the safety and efficacy of combination
therapy with systemic anticoagulation, directed thrombolysis and thrombectomy is unknown.
Case Report: We report a patient with catastrophic CVT due to HIT presenting with
unresponsiveness. CT brain showed intracranial hemorrhage, cerebral edema, cerebral herniation
and extensive cerebral sinus thrombosis. She was treated initially with argatroban and subsequently
had decompressive craniectomy for intracranial hypertension. Cerebral angiography showed
persistent sinus thrombosis despite systemic anticoagulation, endovascular thrombolysis and
mechanical thrombectomy. Continuous intra-sinus alteplase infusion was started concurrently
with systemic argatroban infusion x 24 hours. The patient made a remarkable recovery and achieved
a mRS=1 in 6 months.
Conclusion: Aggressive multimodal therapy with systemic anticoagulation, continuous intra-sinus
thrombolytic infusion, mechanical thrombectomy and neurointensive treatment is a reasonable
management option for patients with catastrophic CVT who failed initial anticoagulation therapy.
Argatroban is an effective alternative when heparin is contraindicated.
Keywords: Cerebral venous thrombosis; Heparin induced thrombocytopenia; Argatroban,
Intrasinus thrombolysis; Mechanical thrombectomy
Introduction
Cerebral venous thrombosis (CVT) affects approximately 5 people per million annually,
representing about 0.5% of all strokes It most commonly affects reproductive aged women [1-4].
The major risk factors include any conditions that promote pro-thrombotic state including genetic
pro-thrombotic diseases, pregnancy, oral contraceptives, malignancy, infection and trauma [1-
5]. Pathogenesis of CVT is due to venous congestion that can cause impaired cerebrospinal fluid
absorption, venous infarction, cerebral edema, and hemorrhage [3].
Headache is the most common symptom of CVT, present in more than 90% of the cases. It
is usually a diffuse progressive headache, however other types of headaches including migrainous
or thunderclap headaches have been reported [1,3-5]. Isolated headache without symptoms of
increased intracranial pressure represent a significant number of the patients [6,7]. Other common
signs and symptoms include papilledema, focal neurological deficit, seizure, altered mental status,
and coma [1,3-5]. Cavernous sinus thrombosis can present with ocular symptoms from cranial
nerve involvement. The superior sagittal and left transverse sinuses are the most frequent site of involvement [1,5].
Neuroimaging is the main mode of diagnosis in CVT. Although
Computed Tomography (CT) is the first line imaging modality in
patients with clinical findings consistent with neurovascular diseases,
it has limited use in diagnosis of CVT. A filling defect known as
the “empty delta sign” was observed with contrast enhanced CT in
28.6% of the cases in one study where it was associated with poor
prognosis [8]. The average delay in diagnosis is 7 days from the onset
of the symptoms, and early diagnosis and treatment is essential in
improving prognosis [4]. The gold standard is a combination of
Magnetic Resonance Imaging (MRI) and Magnetic Resonance
Venography (MRV). Findings equivalent to the “delta sign” can
be observed on MRI, and age of thrombus can be estimated based
on signal characteristics on different sequences. Due to thrombus
age dependent signal intensity, MRI alone may miss the diagnosis
and should be supplemented with MRV when suspicious of sinus
thrombosis [1,3-5,9-11]. Some authors report that MRV is more
sensitive than CTV, while others report that they are equivalent in
detection of the disease. While CTV can be limited by bony artifact
and requires IV contrast and added radiation exposure, MRV is more
sensitive to motion artifact, requires more time to perform, and is
more costly, and less available [9-13]. Although digital subtraction
angiography can aid in the diagnosis and demonstrate additional flow
characteristics of the cerebral circulation, it is neither necessary nor
first line imaging study for the diagnosis, and MRI has the advantage
of differentiating an occluded sinus from congenital hypoplasia [14-
17].
Treatment of CVT usually involves anticoagulation and
management of neurologic sequelae, such as seizure, coma, and
intracranial hypertension, when present. Given the need for
anticoagulation, control of intracranial pressure is primarily
non-surgical. Anticoagulation is necessary to manage the overall
prothrombotic state and to prevent further progression of thrombosis
while physiologic recanalization of the occludedcerebral venous
outflow occurs. Benefits of anticoagulation are weighed against
the risk of expanding intracranial hemorrhage, especially in those
patients with intracranial hemorrhage on presentation. Existing
studies on effectiveness of anticoagulation in the setting of CVT
are limited by their power and study design due to the severity and
rarity of the disease [1,3-5]. Meaningful randomized control trials
would require at least 300 patients, which has not yet been performed
[3]. Although not statistically significant, two existing randomized
controlled trials and various observational trials suggest overall
safety and potential benefits of anticoagulation regardless of presence
or absence of ICH [4,18-26]. Based on this evidence, American
Heart Association/American Stroke Association and The European
Federation of Neurological Societies recommend anticoagulation
with heparin during the acute phase of CVT followed by an oral
Vitamin K antagonist (VKA) for three to six months [5,6]. Although,
intravenousheparin has historically been the primary anticoagulant,
some recent data suggest that low molecular weight heparin
(LMWH) offers equivalent or better treatment, with decreased
hemorrhagic complications, simplified dosing, and less occurrence
of thrombocytopenia [27,28]. A randomized controlled trial of 52
patients revealed no difference in outcome between heparin and
LMWH [29].
Recent concerns over possible publication bias resulting in
underestimation of adverse effect of anticoagulation in treatment
of CVT have surfaced. A comprehensive literature review of studies
published between 1999 and 2013 suggests that anticoagulation with
heparin or LMWH offers statistically significant decrease in mortality
at the cost of increased major bleeding, intracranial hemorrhage
and thrombocytopenia, though authors were not convinced by
the statistical significance due to publication bias of only positive
outcomes [30].
Non-vitamin K antagonist oral anticoagulants (NOAC)
(dabigatran, rivaroxaban, apixaban, edoxaban) and argatroban
(an IV direct thrombin inhibitor) have demonstrated safety and
efficacy in treatment of peripheral Deep Venous Thrombosis (DVT)
and Pulmonary Embolism (PE) with reduced major bleeding risks
compared to VKA. In a small retrospective series, Rivaroxaban was
shown to be effective after initial heparin treatment in the acute phase
of CVT [38]. Other case reports support similar use of rivaroxaban
in patients with antiphospholipid syndrome and Crohn’s diseases
[34,35]. Dabigatran has been shown to be effective and safe in place
of VKA after initial heparin or LWMH treatment in 15 patient case
series [36]. Argatroban was successfully used in a patient with CVT
who was resistant to heparin [39]. Lepirudin was also reported to be
used in a patient with heparin induced thrombocytopenia (HIT) and
CVT [40]. Despite these small series to suggest these drugs are safe
and effective in treatment of CVT [31-40], VKA remains standard of
care for eligible patients. Given the success of these drugs in parallel,
non-cranial disease, and the ongoing development of adequate
reversal agents, further study is warranted to address their in CVT.
Cerebral venous thrombosis remains a dangerous disease,
even with standard therapies. Between 9% to 13% of CVT patients
deteriorate despite anticoagulation treatment. Treatment failure
may result from sub therapeutic anticoagulation, or failure of
anticoagulation despite adequate dosing, where serum assay data is
available [5]. Although no randomized controlled trials have been
performed to demonstrate safety and efficacy of thrombolysis in
CVT, thrombolysis can be considered for patients who are clinically
deteriorating despite adequate anticoagulation treatment [1,3-
5,42,43]. Available thrombolytics include urokinase, streptokinase
and recombinant tissue plasminogen activator (rtPA). A systematic
review of thrombolytics in CVT published between 1966 and 2001
suggested that thrombolytics are reasonably safe. They included 72 studies with a total of 169 patients who were most commonly treated
with locally infused urokinase [44]. Another systematic review of 15
studies with a total of 156 patients published before 2010 showed
that thrombolytics are associated with non-negligible incidence
of major bleeding [45]. A multicenter, prospective, randomized,
open-label, blinded endpoint trial of 164 patients who have poor
prognosis defined by presence of mental status disorder, coma,
intracranial hemorrhagic lesion or thrombosis of the deep cerebral
venous system is currently ongoing [46]. Most studies have used
either anticoagulation or thrombolytics, though there has been a
surge of reported cases where combination of thrombolysis and
anticoagulation were utilized with positive outcomes.
Combination of rtPA with heparin has been used in the past due
to its possible benefit of short half-life of 7-8 minutes, selective clot
lysis compared to other thrombolytics and reduced hemorrhagic risk
[47]. Two studies involving combination therapy showed faster and
more frequent complete recanalization with the combination therapy
compared to that of heparin alone, but it had higher incidence
of bleeding, especially with existing hemorrhage. The study was
also inconsistent in showing a correlation between recanalization
and clinical improvement [48,49]. Similarly, transvenouscatheter
directed thrombolysis with urokinase has been successfully used
with concurrent anticoagulation with heparin [50-53]. Overall,
the combination of thrombolysis with concurrent systemic
anticoagulation is a possible treatment option in patients who are
worsening despite anticoagulation. Clinical judgement must be made
case by case due to lack of quality literature on efficacy and safety of
the treatment.
Mechanical thrombectomy is a final treatment option when
patients are not responsive to anticoagulation and thrombolysis.
A systematic review of literature published between January
1995 and February 2014 where all cases of CVT were treated with
thrombectomy with or without thrombolysis reported an 84% positive
outcome, suggesting that mechanical thrombectomy is a relatively
safe treatment option for patients who failed anticoagulation and
thrombolysis treatment. Although rheolytic catheter thrombectomy
device AngioJet was most commonly used (40%), it was associated
with lower rate of complete recanalization and good outcome [54].
The Merci retrieval device and Penumbra systems are other options,
but evidence of their efficacy and safety are only anecdotal [5]. Balloon
assisted thrombectomy is another option. It is thought to reduce
washout of fibrinolytic agents and occurrence of hemorrhage [5].
Regardless, 10% of the patients had new or worsening intracerebral
hemorrhage, which is a major risk of the treatment.
The largest cohort to date, including 624 CVT patients,
demonstrated an 8% mortality, either from CVT or other causes. Risk
factors associated with poor prognosis were male gender, age over
37 years, coma, mental status change, intracranial hemorrhage on
admission, thrombosis of the deep cerebral venous system, central
nervous system infection and cancer [4]. We report a patient with
hemorrhagic presentation of cerebral venous sinus thrombosis
secondary to heparin-induced thrombocytopenic thrombosis (HITT)
after craniotomy, with rapid clinical deterioration to deep coma
despite systemic anticoagulation, who made a remarkable recovery
after combination mechanical thrombectomy, continuous intrasinus
tPA infusion, and concurrent systemic argatroban infusion.
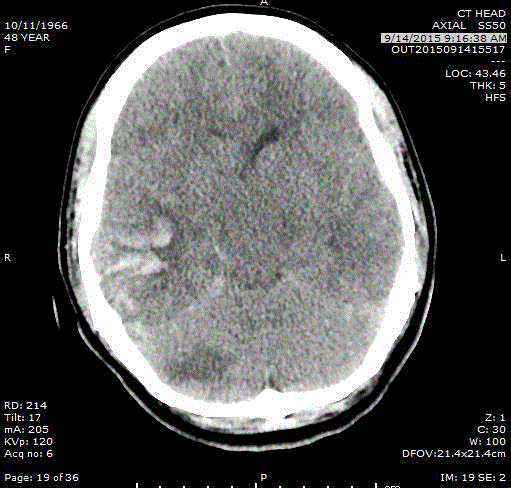
Figure 1
Figure 2
Figure 2
MR angiogram showing absent signal along superior sagittal ,
transverse and sigmoid sinus on the right.
Case
A 48 year old previously healthy female presented to a separate
institution with nausea and headaches one year status post status
post cyber knife surgery of a 4.2 cm parafalcine meningioma. MR
imaging evaluation showed increased cerebral edema around the
treated meningioma, and she was admitted for steroid treatment
and observation. Five days after admission, she became acutely
unresponsive and underwent emergent CT imaging demonstrating
an acute right cerebellar ischemic infarct and a right temporal
intraparenchymal hemorrhage (Figure 1), for which she underwent
emergent right temporal craniotomy for hematoma evacuation.
Postoperatively, she remained intubated, opening her eyes to noxious
stimulation, localizing with her right upper extremity, withdrawing
her lower extremity, and hemiplegic on the left.
A post-operative head CT without contrast revealed only expected
post-surgical changes, and MRI/MR Angiography (Figure 2) revealed
superior sagittal sinus thrombosis extending into the right transverse,
sigmoid and internal jugular vein, with evidence of early restricted
diffusion without FLAIR changes in the bilateral parietal and temporal
cerebral cortex concerning for developing venous infarction. She
was started on heparin drip and transferred to our institution for
higher level of care. Given the acuity of onsent, MRI findings, and
her poor neurological exam despite anticoagulation, she was taken
for emergent transfemoral cerebral angiogram and transvenous
mechanical thrombectomy and thrombolysis of the superior sagittal
sinus, right transverse sinus, sigmoid sinus, and right jugular vein
with aspiration thrombectomy followed by infusion of 6 mg of tPA
into the superior sagittal sinus. Continuous tPA infusion into the
cerebral venous sinus system was not performed at that time because
of concern for hemorrhagic complications related to craniotomy
performed earlier the same day. Flow was successfully reestablished
at the time of thrombectomy and she was transported back to the
neurosurgical ICU where an intraparenchymal monitor (Licox) was
placed without complication. Serum laboratory evaluation revealed
thrombocytopenia, and all heparin products were discontinued and
anticoagulation was transitioned to argatroban.
Unfortunately, 3 days after endovascular treatment, she
developed elevated intracranial pressure and deterioration of
neurological examination to include loss of all motor function and
brainstem reflexes, despite therapeutic anticoagulation. Emergent non contrast repeat head CT demonstrated new hyperdensity
consistent with recurrent cerebral venous sinus occlusion, without
radiographic evidence of new or worsening hemorrhagic or
ischemic infarction. She was taken for emergent repeat endovascular
treatment, repeat transfemoral diagnostic cerebral angiography
and transvenous thrombectomy, followed by placement of a micro
catheter in the posterior superior sagittal sinus for continuous tPA
infusion. She received10 mg intra-sinus bolus of tPA followed by
50 mg infusion over 3 hours followed by 2.5mg/hr continuous
infusion of intravenous sinus tPA. The tPA infusion was continued
for 24 hours and maintained concurrently with argatroban infusion
for therapeutic anticoagulation. A CT scan of the brain post sinus
catheter placement during infusion did not demonstrate hemorrhagic
or ischemic complications.
Her cerebral edema, intracranial hypertension, and venous
infarction were managed with hypertonic saline, mannitol, and
dexamethasone, and she underwent continuous EEG monitoring
in the ICU for concern of subclinical status epilepticus. Her mean
arterial pressure was supported with vasopressors to maintain
cerebral perfusion. Her clinical course was further complicated by
sepsis and electrographic evidence of seizure managed with antibiotic
therapy and antiepileptic mediations. The argatroban was ultimately
transitioned to coumadin with INR goal between 2-3. She eventually
underwent tracheostomy and PEG tube placement. After inpatient
rehabilitation, she was eventually discharged back to home. On
her most recent follow up at 1 year post-discharge, she has a near
normal neurological exam, living independently, with a persistent
lefthomonymous hemianopsia, and mRS of 1.
Discussion
Systemic anticoagulation is the primary treatment of CVT;
however, thrombolysis and thrombectomy are indicated in cases of
severe neurologic compromise and / or where anticoagulation does
not result in clinical improvement. The main risk of treatment in
CVT is major intracerebral hemorrhage, and benefits of any of the
treatments are yet to be supported by randomized controlled trials
[1-4]. Thus, more data on treatments of CVT is necessary to develop
a treatment algorithm for CVT.
We present a case of CVT that was managed with concurrent
systemic anticoagulation with argatroban and continuous catheter
directed infusion of tPA, in addition to thrombectomy. Two
unique aspects of our case are use of argatroban as an anticoagulant
during an acute phase of CVT, and the concurrent use of systemic
anticoagulation and intracerebral sinus tPA infusion.
NOACs have been used in treatment of CVT in place of VKA
after initial heparin therapy based on case reports [31-38], but reports
of their use as the primary anticoagulant during the acute phase of
CVT are limited. There is only single reported use of NOACs in place
of heparin and LMWH in acute phase of CVT in a patient who was
resistant to heparin [39]. Our case is the second reported case with
use of argatroban as an anticoagulant during an acute phase of CVT.
These two cases show some evidence that argatroban is an alternate
agent for systemic anticoagulation in the setting of heparin resistance
or HIT.
There are two studies involving combination therapy of systemic
anticoagulation and thrombolysis. The first study included 9 patients
who received transfemoral direct thrombolysis treatment with a rapid
injection of 10 mg alteplase followed by continuous infusion until
complete recanalization or a total dose of 100 mg per reached. The
procedure was repeated if complete recanalization did not occur. The
range of a total alteplase dosage was between 50 mg to 300 mg with
average of 135 mg. Four patients received initial heparin treatment,
while all 9 patients received post-procedure warfarin treatment,
which 7 of them continued for 3 months. During the treatment, two
patients had oozing at a puncture site and intraperitoneal hematoma.
All patients have shown some level of clinical improvement posttreatment
[48].
The second study included 12 patients, 7 of whom had some
type of hemorrhage somewhere in their the body, who received
transfemoral direct thrombolysis with rtPA with a loading dose of
1mg/cm followed by continuous direct infusion at 1 mg/h to 2 mg/h
until flow was restored with concurrent IV heparin dosed to reach
PTT twice the control value. A total rtPA dose ranged between 23 mg
to 128 mg with average of 46 mg. Symptoms improved in all patients
except three, with one not improving and two patients having
worsening hemorrhage. Although most patients improved clinically,
already existing hemorrhage worsened with treatment, limiting its
safety in this population [49].
Similarly, transfemoral direct thrombolysis with urokinase has
been successfully used with concurrent anticoagulation with heparin
with PTT twice the control value [50-53]. The first study included
five patients who were treated with continuous urokinase infusion
of 3,500 units/kg/hr for six to nine hours following 7,500 units of
IV heparin, and then the combination therapy (30,000 units/day to
40,000 units/day by continuous IV heparin; urokinase, 3,000 units/
kg every six to eight hours) was continued for 2-6 days [50]. The
second study included seven patients who were treated with constant
urokinase infusion through peripheral IV with total infusion times
ranging from 88 to 244 hours (average 163 hours) while being
anticoagulated with IV heparin with PTT 1.5 to 2 times the control.
The loading dose ranged between 80,000 and 250,000 [51].
Another case reported administration of 1,50,000 unit bolus
of urokinase into the straight sinus followed by 1,00,000 unit/hr
infusion twice 9 hrs apart while systemically anticoagulated with
heparin. Infusion was stopped due to spontaneous retroperitoneal
and psoas muscle hematoma after 20 hrs of treatment [52]. The last
case reported an initial bolus of 250,000 U of urokinase into superior
sagittal sinus in a pulse-spray manner over 2 hours followed by a
constant infusion at a rate of 80,000 U/h for total of 165 hours, with
a total urokinase dose of 13.79 million units [53]. The patient was
concurrently systemically anticoagulated with IV heparin with a
loading dose of 5000 U and a maintenance dose of 1000 U/h [53].
Our case along with other reported studies show that
the combination of thrombolysis with concurrent systemic
anticoagulation is a reasonable treatment option for patients who
worsening despite anticoagulation. Our experience also shows that
argatroban is a reasonable alternative to heparin for anticoagulation
when heparin is contraindicated.
References
- Bousser MG, Ferro JM. Cerebral venous thrombosis: An update. Lancet Neurol. 2007;6(2):162-170.
- Stam J. Cerebral venous and sinus thrombosis: Incidence and causes. Adv Neurol. 2003;92:225-232.
- Stam J. Thrombosis of the cerebral veins and sinuses. N Engl J Med. 2005;352(17):1791-1798.
- Ferro JM, Canhao P, Stam J, Bousser MG, Barinagarrementeria F, ISCVT Investigators. Prognosis of cerebral vein and dural sinus thrombosis: Results of the international study on cerebral vein and dural sinus thrombosis (ISCVT). Stroke. 2004;35(3):664-670.
- Saposnik G, Barinagarrementeria F, Brown RD Jr, Bushnell CD, Cucchiara B, Cushman M, et al. Diagnosis and management of cerebral venous thrombosis: A statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42(4):1158-1192.
- Einhäupl K1, Stam J, Bousser MG, De Bruijn SF, Ferro JM, Martinelli I, et al. EFNS guideline on the treatment of cerebral venous and sinus thrombosis in adult patients. Eur J Neurol. 2010;17(10):1229-1235.
- Cumurciuc R, Crassard I, Sarov M, Valade D, Bousser MG. Headache as the only neurological sign of cerebral venous thrombosis: A series of 17 cases. J Neurol Neurosurg Psychiatry. 2005;76(8):1084-1087.
- Virapongse C, Cazenave C, Quisling R, Sarwar M, Hunter S. The empty delta sign: Frequency and significance in 76 cases of dural sinus thrombosis. Radiology. 1987;162(3):779-785.
- Hinman JM, Provenzale JM. Hypointense thrombus on T2-weighted MR imaging: A potential pitfall in the diagnosis of dural sinus thrombosis. Eur J Radiol. 2002;41(2):147-152.
- Dormont D, Anxionnat R, Evrard S, Louaille C, Chiras J, Marsault C. MRI in cerebral venous thrombosis. J Neuroradiol. 1994;21(2):81-99.
- Leach JL, Fortuna RB, Jones BV, Gaskill-Shipley MF. Imaging of cerebral venous thrombosis: Current techniques, spectrum of findings, and diagnostic pitfalls. Radiographics. 2006;26 Suppl 1:3.
- Ozsvath RR, Casey SO, Lustrin ES, Alberico RA, Hassankhani A, Patel M. Cerebral venography: Comparison of CT and MR projection venography. AJR Am J Roentgenol. 1997;169(6):1699-1707.
- Casey SO1, Alberico RA, Patel M, Jimenez JM, Ozsvath RR, Maguire WM, et al. Cerebral CT venography. Radiology. 1996;198(1):163-170.
- Isensee C, Reul J, Thron A. Magnetic resonance imaging of thrombosed dural sinuses. Stroke. 1994;25(1):29-34.
- Vogl TJ, Bergman C, Villringer A, Einhaupl K, Lissner J, Felix R. Dural sinus thrombosis: Value of venous MR angiography for diagnosis and follow-up. AJR Am J Roentgenol. 1994;162(5):1191-1198.
- Rodallec MH, Krainik A, Feydy A, Hélias A, Colombani JM, Jullès MC, et al. Cerebral venous thrombosis and multidetector CT angiography: Tips and tricks. Radiographics. 2006;26 Suppl 1:3.
- Linn J, Ertl-Wagner B, Seelos KC, et al. Diagnostic value of multidetector-row CT angiography in the evaluation of thrombosis of the cerebral venous sinuses. AJNR Am J Neuroradiol 2007;28(5):946-952.
- Breteau G, Mounier-Vehier F, Godefroy O, et al. Cerebral venous thrombosis 3-year clinical outcome in 55 consecutive patients. J Neurol 2003;250(1):29-35.
- Preter M, Tzourio C, Ameri A, Bousser MG. Long-term prognosis in cerebral venous thrombosis. follow-up of 77 patients. Stroke 1996;27(2):243-246.
- Wingerchuk DM, Wijdicks EF, Fulgham JR. Cerebral venous thrombosis complicated by hemorrhagic infarction: Factors affecting the initiation and safety of anticoagulation. Cerebrovasc Dis 1998;8(1):25-30.
- Brucker AB, Vollert-Rogenhofer H, Wagner M, et al. Heparin treatment in acute cerebral sinus venous thrombosis: A retrospective clinical and MR analysis of 42 cases. Cerebrovasc Dis 1998;8(6):331-337.
- Bousser MG, Chiras J, Bories J, Castaigne P. Cerebral venous thrombosis--a review of 38 cases. Stroke 1985;16(2):199-213.
- Ferro JM, Correia M, Pontes C, Baptista MV, Pita F, Cerebral Venous Thrombosis Portuguese Collaborative Study Group (Venoport). Cerebral vein and dural sinus thrombosis in portugal: 1980-1998. Cerebrovasc Dis 2001;11(3):177-182.
- Stam J, De Bruijn SF, DeVeber G. Anticoagulation for cerebral sinus thrombosis. Cochrane Database Syst Rev 2002;(4)(4):CD002005.
- de Bruijn SF, Stam J. Randomized, placebo-controlled trial of anticoagulant treatment with low-molecular-weight heparin for cerebral sinus thrombosis. Stroke 1999;30(3):484-488.
- Einhaupl KM, Villringer A, Meister W, et al. Heparin treatment in sinus venous thrombosis. Lancet 1991;338(8767):597-600.
- Coutinho JM, Ferro JM, Canhao P, et al. Unfractionated or low-molecular weight heparin for the treatment of cerebral venous thrombosis. Stroke 2010;41(11):2575-2580.
- Misra UK, Kalita J, Chandra S, Kumar B, Bansal V. Low molecular weight heparin versus unfractionated heparin in cerebral venous sinus thrombosis: A randomized controlled trial. Eur J Neurol 2012;19(7):1030-1036.
- Afshari D, Moradian N, Nasiri F, Razazian N, Bostani A, Sariaslani P. The efficacy and safety of low-molecular-weight heparin and unfractionated heparin in the treatment of cerebral venous sinus thrombosis. Neurosciences (Riyadh) 2015;20(4):357-361.
- Cundiff DK. Anticoagulants for cerebral venous thrombosis: Harmful to patients? Stroke 2014;45(1):298-304.
- Bacchus F, Schulman S. Clinical experience with the new oral anticoagulants for treatment of venous thromboembolism. ArteriosclerThrombVasc Biol 2015;35(3):513-519.
- Fesler MJ, Creer MH, Richart JM, et al. Heparin-induced thrombocytopenia and cerebral venous sinus thrombosis: Case report and literature review. Neurocrit Care 2011;15(1):161-165.
- King AB, O'Duffy AE, Kumar AB. Heparin resistance and anticoagulation failure in a challenging case of cerebral venous sinus thrombosis. Neurohospitalist 2016;6(3):118-121.
- Sugie M, Iizuka N, Shimizu Y, Ichikawa H. Cerebral venous thromboembolism in antiphospholipid syndrome successfully treated with the combined use of an anti-xa inhibitor and corticosteroid. Intern Med 2015;54(23):3051-3051-6.
- Young-Hak Cho, Min Kyu Chae, Jae Myung Cha, Joung Il Lee, Kwang Ro Joo, Hyun Phil Shin, et al. Cerebral venous thrombosis in a patient with crohn's disease. Intest Res. 2016;14(1):96-101.
- Mendonca MD, Barbosa R, Cruz-e-Silva V, Calado S, Viana-Baptista M. Oral direct thrombin inhibitor as an alternative in the management of cerebral venous thrombosis: A series of 15 patients. Int J Stroke. 2015;10(7):1115-8.
- Patel SI, Obeid H, Matti L, Ramakrishna H, Shamoun FE. Cerebral venous thrombosis: Current and newer anticoagulant treatment options. Neurologist. 2015;20(5):80-8.
- Geisbusch C, Richter D, Herweh C, Ringleb PA, Nagel S. Novel factor xa inhibitor for the treatment of cerebral venous and sinus thrombosis: First experience in 7 patients. Stroke. 2014;45(8):2469-71.
- King AB, O'Duffy AE, Kumar AB. Heparin resistance and anticoagulation failure in a challenging case of cerebral venous sinus thrombosis. Neurohospitalist. 2016;6(3):118-21.
- Refaai MA, Warkentin TE, Axelson M, Matevosyan K, Sarode R. Delayedonset heparin-induced thrombocytopenia, venous thromboembolism, and cerebral venous thrombosis: A consequence of heparin "flushes". ThrombHaemost. 2007;98(5):1139-40.
- James RF, Palys V, Lomboy JR, Lamm JR, Simon SD. The role of anticoagulants, antiplatelet agents, and their reversal strategies in the management of intracerebral hemorrhage. Neurosurg Focus. 2013;34(5):E6.
- Bousser MG. Cerebral venous thrombosis: Nothing, heparin, or local thrombolysis?. Stroke. 1999;30(3):481-3.
- Benamer HT, Bone I. Cerebral venous thrombosis: Anticoagulants or thrombolyic therapy? J NeurolNeurosurg Psychiatry. 2000;69(4):427-30.
- Canhao P, Falcao F, Ferro JM. Thrombolytics for cerebral sinus thrombosis: A systematic review. Cerebrovasc Dis. 2003;15(3):159-66.
- Dentali F, Squizzato A, Gianni M, De Lodovici ML, Venco A, Paciaroni M, et al. Safety of thrombolysis in cerebral venous thrombosis. A systematic review of the literature. ThrombHaemost. 2010;104(5):1055-62.
- Coutinho JM, Ferro JM, Zuurbier SM, Mink MS, Canhão P, Crassard I, et al. Thrombolysis or anticoagulation for cerebral venous thrombosis: Rationale and design of the TO-ACT trial. Int J Stroke. 2013;8(2):135-40.
- Bousser MG. Cerebral venous thrombosis: Nothing, heparin, or local thrombolysis?. Stroke. 1999;30(3):481-3.
- Kim SY, Suh JH. Direct endovascular thrombolytic therapy for dural sinus thrombosis: Infusion of alteplase. AJNR Am J Neuroradiol. 1997;18(4):639- 45.
- Frey JL, Muro GJ, McDougall CG, Dean BL, Jahnke HK. Cerebral venous thrombosis: Combined intrathrombusrtPA and intravenous heparin. Stroke. 1999;30(3):489-94.
- Di Rocco C, Iannelli A, Leone G, Moschini M, Valori VM. Heparinurokinase treatment in aseptic dural sinus thrombosis. Arch Neurol. 1981;38(7):431-5.
- Smith TP, Higashida RT, Barnwell SL, Halbach VV, Dowd CF, Fraser KW, et al. Treatment of dural sinus thrombosis by urokinase infusion. AJNR Am J Neuroradiol. 1994;15(5):801-7.
- Holder CA, Bell DA, Lundell AL, Ulmer JL, Glazier SS. Isolated straight sinus and deep cerebral venous thrombosis: Successful treatment with local infusion of urokinase. case report. J Neurosurg. 1997;86(4):704-7.
- Rael JR, Orrison WW, Baldwin N, Sell J. Direct thrombolysis of superior sagittal sinus thrombosis with coexisting intracranial hemorrhage. AJNR Am J Neuroradiol. 1997;18(7):1238-42.
- Siddiqui FM, Dandapat S, Banerjee C, Zuurbier SM, Johnson M, Stam J, et al. Mechanical thrombectomy in cerebral venous thrombosis: Systematic review of 185 cases. Stroke. 2015;46(5):1263-8.