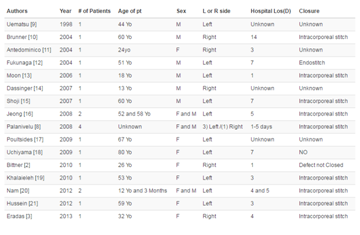
Research Article
Propionibacterium acnes Infection of a Mitral Annuloplasty Ring
Yaping Xu1*, Chenxue Jiang1, Gang Lin1, Xiang Zhu2, Shuiyun Han2, Xiaojiang Sun2, Jinshi Liu3, Qixun Chen3 and Weimin Mao3
1Department of Radiation Oncology, Zhejiang Cancer Hospital, China
2First Clinical Medical School, Wenzhou Medical University, China
3Department of Thoracic Surgery, Zhejiang Cancer Hospital, China
*Corresponding author: Yaping Xu, Department of Radiation Oncology, Zhejiang Cancer Hospital, No.1 Banshan East Road, Hangzhou, China
Published: 03 Jan, 2018
Cite this article as: Xu Y, Jiang C, Lin G, Zhu X, Han S,
Sun X, et al. Trimodality versus Chemo
Radiation Therapy Alone: The Role
of Surgery in Treatment of Locally
Advanced Squamous Cell Carcinoma
of the Esophagus. Ann Clin Case Rep.
2018; 3: 1490.
Abstract
Background and Purpose: The current practice varies in treatments of locally advanced Squamous
Cell Carcinoma (SCC) of esophagus in different countries. This study aimed to compare the results
of trimodality therapy with CRT alone in patients with locally advanced resectable SCC.
Patients and Methods: Patients with locally advanced resectable SCC of esophagus were eligible.
For trimodality, patients received surgery and preoperative/postoperative chemoradiation. In
CRT alone group, patients only received radiation and chemotherapy. Local tumor control, 3-year
survival and treatment-related mortality were assessed.
Results: 184 consecutive patients were analyzed. 109 were treated with trimodality therapy, 75
received CRT alone depended on patients’ willing. 17.4% of the resected patients in trimodality
group had locoreginal recurrent disease versus 30.7% in the CRT alone group (P=0.036). The 3-year
progression-free survival (PFS) was 53.8% versus 33.5% (P=0.019), and the overall survival (OS)
was 51.2% versus 39.8% (P=0.011), for patients received trimodality and CRT alone, respectively.
Treatment-related mortality was 3.7% in trimodality group compared with 1.3% in definitive CRT
group (P=0.650). There was no significant difference in the 3-year OS in patients receiving a 50.4
Gy radiation dose compared with >50.4 Gy radiation dose in CRT alone group (45.3% vs. 36.4%, P
=0.927).
Conclusions: Compared with CRT alone, trimodality therapy appeared to have superior local
control, PFS and OS, with similar treatment-related mortality for the treatment of patients with
SCC of esophagus. The role of surgery could not be replaced by CRT alone, even with increased
radiation dose.
Keywords: Oesophageal squamous cell carcinoma; Trimodality therapy; Preoperative chemoradiotherapy; Postoperative chemoradiotherapy; Definitive chemoradiotherapy
Introduction
Esophageal cancer is the sixth most common cause of cancer deaths worldwide and is more
common in the developing nations [1]. Esophageal cancers are histologically classified as Squamous
Cell Carcinoma (SCC) or adenocarcinoma. SCC is the major histology in Eastern Europe and Asia,
and 95% of esophageal cancer is pathologically diagnosed as SCC in China [2]. Radiochemotherapy
and surgical resection are standard therapies for patients with locally advanced resectable SCC of
the esophagus.
Numerous randomized trials have investigated the impact of radiotherapy dose modifications,
combined-modality therapy, and preoperative/postoperative administration of adjuvant therapy
in an effort to improve effectiveness without compromising safety, reducing the incidence
of local recurrence, and prolonging survival [3-7]. For example, results from the multicenter phase III randomized trial (CROSS study), the largest trial in its class, showed that preoperative
chemoradiotherapy (CRT) with carboplatin and paclitaxel significantly improved overall survival
(OS) and disease-free survival (DFS) compared to surgery alone in patients with resectable (T2-
3, N0-1, M0) esophageal or esophagogastric junction (EGJ) cancers [8]. Similarly, the results of two meta-analyses have shown that preoperative CRT combined
with surgery significantly reduced 3-year mortality and locoregional
recurrence when compared with surgery alone [9,10]. On the other
hand, the efficacy of postoperative CRT compared to surgery alone
has not been demonstrated in a randomized trial in patients with
esophageal cancer. However, in retrospective analyses, the addition
of postoperative CRT has been associated with survival benefit in
patients with locally advanced esophageal cancer, such as those that
are lymph node-positive and with deeper primary tumor invasion
(pT3, pT4), compared with surgery alone [11-13]. Finally, two
randomized trials comparing trimodality therapy with definitive CRT
demonstrated no OS benefit with the addition of esophagectomy to
CRT [14,15], especially in patients with locally advanced SCC of the
esophagus who experience response to initial CRT [15]. Therefore,
the optimal multimodality therapy for locally advanced SCC of the
esophagus is still unclear.
This retrospective study was designed to determine the best
approach to administer multimodality therapy to patients with locally
advanced SCC of the esophagus. The primary aim was to determine
if the addition of surgical resection to CRT decreases local recurrence
and prolongs in survival compared with CRT alone. Furthermore, we
sought to assess the factors that may affect survival and recurrence in
patients with locally advanced SCC of the esophagus.
Materials and Methods
Patient population
Patients with locally advanced resectable (cT3, potentially
resectable cT4 or N+) SCC of the esophagus who were treated
with preoperative CRT or postoperative CRT plus esophagectomy
(trimodality therapy group), or only radiation with chemotherapy
(CRT alone group), between April 2011 and November 2015 were
included. Patients were included if they were aged 18 to 75 years, with
an ECOG/WHO performance score <2, and showed <10% weight loss.
Patients were excluded if their esophageal cancer was located in the
cervical esophagus, if this was their second malignancy, if they were
identified as receiving irradiation to a site other than the esophagus,
or if they received radiotherapy without concurrent chemotherapy.
The patient population consisted partially of patients enrolled in
the ZTOG1201 trial, a randomized controlled trial in which eligible
patients were randomly assigned to receive preoperative CRT plus
surgery or surgery plus postoperative CRT (NCT01463501) [16].
Chemoradiotherapy
Chemotherapy consisted of concurrent paclitaxel (50 mg/m2 of
body-surface area) and carboplatin (intravenous carboplatin [AUC 2
mg/ml per min]) targeted at an area under the curve of two, starting
on days 1, 8, 15, 22, 29, and 36 during the first and sixth weeks of
radiotherapy in the preoperative CRT group and the CRT alone
group. Patients in the postoperative CRT group mostly underwent
postoperative CRT at 4–6 weeks after surgery. Chemotherapy in
the postoperative CRT group consisted of two cycles of sequential
paclitaxel (150 mg/m2 of body-surface area) and carboplatin
(intravenous carboplatin [AUC 5 mg/mL per min]) targeted at an
area under the curve of five, starting on week 3, and week 6 after
radiotherapy.
All patients were treated with external-beam radiation using
an intensity-modulated radiation therapy technique. Gross tumor
volume was drawn on each relevant slice of the planning CT and
was defined by the primary tumor and any enlarged regional lymph nodes. The planning target volume (PTV) provided a proximal and distal margin of 4 cm and a radial margin of 1.3 cm around the
gross tumor volume. Individually shaped beams were used in each
field by multileaf collimators to ensure optimal sparing of normal
tissue. The daily prescription dose of 1.8 to 2.0 Gy was specified by
the International Commission on Radiation Units and Measurement
50/62 reference point, and the 95% isodose had to encompass the
entire PTV. The maximum dose to the PTV was not to exceed the
prescription dose by <7%. Tissue density inhomogeneity correction
was used.
Patients were required to have complete information regarding
the total radiation planning, as well as the chemotherapy regimen.
We limited our analysis to patients who received radiation doses of
41.4 to 50.4 Gy in the preoperative CRT group, 45 to 50.4 Gy in the
postoperative CRT group, and 50 to 64.8 Gy in the definitive CRT
group, as these represent the expected ranges for preoperative/
postoperative to definitive radiation doses. All patients received
the same chemotherapy regimen with paclitaxel and carboplatin.
The delivery of concurrent CRT was determined by only including
patients who were identified as receiving their chemotherapy within a
1-week window before or after the initiation of radiotherapy.
Surgery
Patients in the trimodality therapy group preferably underwent
surgery at 4-6 weeks after completion of preoperative CRT, or as
soon as possible after randomization in postoperative subgroup.
The final choice of surgical procedure including minimally invasive
oesophagectomy (MIE) or open oesophagectomy (OE) with a
intrathoracic gastric tube reconstruction (Ivor Lewis procedure)
or neck anastomosis (Mckeown procedure) was at the surgeon’s
discretion, depending on tumor localization, patient characteristics.
Gastric-tube reconstruction with a cervical anastomosis was the
preferred technique. A wide local excision of the N1 lymph nodes,
including standard excision of the celiac nodes, was carried out.
Assessment of recurrence
Relapses were classified as locoregional or distant. Locoregional
relapses were defined as recurrences at the site of the primary tumor
or locoregional lymph nodes. Lymph node recurrences at the celiac
trunk or in the supraclavicular region were also considered to be
locoregional. Distant recurrences were defined as non-regional
lymph node recurrences, systemic metastases, malignant pleural
effusions, or peritoneal metastases. Most patients suspected of
experiencing recurrence underwent a CT scan of the thorax and
abdomen or an endoscopy. If necessary, cytology or histology was
obtained. If a second recurrence was detected within 4 weeks after the
first occurrence, it was considered to be synchronous. Localization
and date of identification of all locoregional and distant recurrences
were recorded.
Statistical analysis
Demographic details were compared between patients who
received trimodality treatment or CRT alone using the χ2 test, the
Fisher exact test, and the Mann-Whitney test, where appropriate.
Multivariate Cox regression of OS and tumor recurrence was
performed to calculate hazard ratios (HRs) and 95% confidence
intervals (CIs) using these same covariates, excluding age, gender,
WHO/ECOG score, tumor location, tumor length, T staging, N
staging, radiation dose, and treatment approach. Kaplan-Meier
analyses of OS were performed comparing patients who received definitive radiation to a dose of 50 to 50.4 Gy with those who received >50.4 Gy. Survival analyses were also performed comparing those
who received definitive CRT with those who received trimodality
therapy after stratification by treatment sequence (preoperative CRT
or postoperative CRT). Significant values were defined as those with
a P-value of <0.05.
Results
Study population
The analysis included 184 patients: 109 (59.2%) underwent
trimodality therapy, including 57 (31.0%) who underwent
preoperative CRT followed by oesophagectomy and 52 (28.2%) who
underwent oesophagectomy followed by postoperative CRT, and
75 (40.8%) underwent CRT alone. The median age was 60 years old
(range, 41 to 75 years). Patients undergoing trimodality therapy were
more likely to be younger, male gender, have a longer tumor length,
and a tumor location in the distal third of the esophagus. Additional
details on patient characteristics are listed in Table 1.
Analysis of recurrence
After a median follow-up of 36 months (range, 6 to 53 months)
and a median survival time of 31 months (95% CI: 20.2–41.8 months), 33.9% (37/109) of the resected patients in the trimodality
therapy group had recurrent disease versus 50.7% (38/75) in the
CRT alone group. There was a significant difference between the two
treatment groups in terms of tumor recurrence (P = 0.023). Only
17.4% (19/109) of the resected patients in the trimodality arm had
locoregional recurrent disease versus 30.7% (23/75) in the definitive
CRT arm (P = 0.036). Moreover, fewer patients (16.5%, 18/109) in
the trimodality arm had distant failure compared to those in the CRT
alone arm (18.7%, 14/75), although this difference was not significant
(P = 0.705).
Table 2 lists the univariate and multivariate Cox Regression
Analyses for tumor recurrence. Prognostic factors predicting
locoregional relapses in univariate analysis were younger age and CRT
alone. In the multivariate analysis, the backward method showed that
patients with younger age who received CRT alone had a significantly
increased risk of developing a locoregional relapse.
Survival outcomes
A significant difference in the 3-year progression-free survival
(PFS) was observed between the trimodality group and the CRT alone
group (53.8%, 95% CI: 42.8-64.8% vs. 33.5%, 95% CI: 20.6-46.4%,
respectively; P = 0.019). Figures 1A, 1B, and 1Cshow the differences between the trimodality therapy group and CRT alone group for PFS(53.8%, 95% CI: 42.8-60.8% vs. 33.5%, 95% CI: 20.6-46.4%; P =
0.019),locoregional PFS(65.7%, 95% CI: 54.9-76.5% vs. 47.0%, 95%
CI: 32.3-61.7%; P= 0.040), and distant PFS (73.5%, 95% CI: 63.3-
83.7% vs. 66.7%, 95% CI: 53.2-80.2%; P = 0.380), respectively.
Trimodality treatment was also associated with superior OS
outcomes. In the trimodality treatment group, the 3-year OS rate was
51.2% (95% CI: 36.1-66.3%) and the median survival time was not
reached; while in the CRT alone group, the 3-year OS rate was 39.8% (95% CI: 26.7-
52.9%) with a median survival time of 22.0 months
(95%: CI 17.1 to 26.9 months; P = 0.011, (Figure 2)). Furthermore,
treatment related mortality was 3.7% in the trimodality group
compared with 1.3% in the definitive CRT group (P = 0.650).
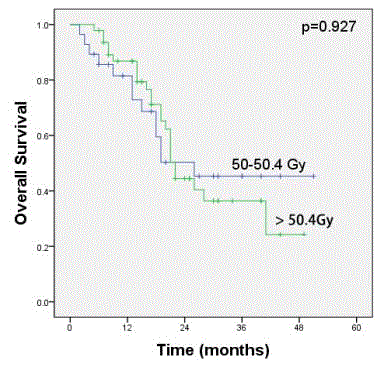
A total of 37.3% (28/75) of patients received a radiation dose of
50 to 50.4 Gy, and 62.7% (47/75) received >50.4 Gy radiation dose
in CRT alone group. These who received 50 to 50.4 Gy had a median
OS of 26.0 months (95% CI: not reached) and a 3-year OS of 45.3%
(95% CI: 24.7-65.9%). However, the survival was not significantly different (P= 0.927) to those receiving radiation doses >50.4 Gy, who
had a median survival of 22.0 months (95% CI: 20.2-23.8 months)
and a 3-year OS of 36.4% (95% CI: 19.4-53.1%). Figure 3 depicts the
Kaplan-Meier survival curves for patients who received CRT alone
by doses. In multivariate analysis, an increased radiation dose was
not associated with OS: only younger age was associated with inferior
survival (HR= 2.941; 95% CI: 1.441-5.988; P = 0.003). Further details
of the multivariate analysis are shown in Table 3.
Table 1
Figure 1
Figure 1
Kaplan-Meier survival curves for patients receiving trimodality therapy or CRT alone: A. There was significant difference between the groups in the 3-year
progression-free survival (53.8%, 95% CI: 42.8–60.8% vs. 33.5%, 95% CI: 20.6–46.4%; P = 0.019); B. There was significant difference between the groups in the
3-year locoregional progression-free survival (65.7%, 95% CI: 54.9–76.5% vs. 47.0%, 95% CI: 32.3–61.7%; P = 0.040); C. There was no significant difference
between the groups in the 3-year distant progression-free survival (73.5%, 95% CI: 63.3–83.7% vs. 66.7%, 95% CI: 53.2–80.2%; P = 0.380).
Figure 2
Figure 2
Kaplan-Meier survival curves for patients receiving trimodality
therapy or CRT alone. There was significant difference between the groups
in the 3-year overall survival (51.2%, 95% CI: 36.1–66.3% vs. 39.8%, 95%
CI: 26.7–52.9%; P = 0.011).
Figure 3
Figure 3
Kaplan-Meier survival curves for patients receiving a 50.4 Gy
radiation dose or >50.4 Gy radiation dose in CRT alone group. There was
no significant difference in the 3-year overall survival (45.3%, 95% CI: 24.7–
65.9% vs. 36.4%, 95% CI: 19.4–53.1%; P = 0.927).
Discussion
The present study indicated that adding surgery to CRT for the
treatment of clinical resectable, locally advanced SCC of the esophagus
significantly decreased the local recurrence rates, prolonged PFS
and OS, with similar treatment-related mortality for the treatment
compared with CRT alone. In the trimodality treatment group,
the 3-year OS was 51.2% (95% CI: 36.1-66.3%) similar to the result
achieved for preoperative CRT in the CROSS trial (51%) [8], which
were better than that achieved in the CRT alone group (39.8%, 95%
CI: 26.7-52.9%).
Surgery is a major component of treatment for resectable disease.
Based on data from the National Cancer Database of America,
trimodality therapy was associated with improved OS (P < 0.001),
with a median OS of 35.6 months and 3-year OS of 49.6%, compared to patients receiving CRT (median and 3-year OS were 16.8 months
and 26.8%, respectively) [17]. The effect of adding surgery to CRT
in patients with locally advanced SCC of esophagus has also been
evaluated in two randomized trials [14,15]. Stahl et al. randomized
172 patients to either induction chemotherapy followed by CRT and
surgery or induction chemotherapy followed by CRT [14]. The 2-year
PFS rate was better in the surgery group (64.3%) than in the CRT
group (40.7%), without significantly affecting 3-year OS (31% vs. 24%,
respectively). However, this study was prematurely terminated due to
lack of accrual. On the other hand, Bedenne et al. (FFCD 9102 trial)
showed that adding surgery to CRT provided no benefit compared
with treatment with additional CRT, especially in patients with locally
advanced SCC of the esophagus who responded to initial CRT [15].
However, this trial suffered from suboptimal study design, indeed, the
results of non-randomized patients in the FFCD 9102 phase III trial
indicated that OS did not differ between responders to induction CRT
and patients having salvage surgery after clinical failure of CRT [18].
Moreover, in a recent prospective study that compared the outcomes
of surveillance versus surgical resection in patients with esophageal
cancer achieving complete clinical response after preoperative CRT,
surgical resection was independently associated with less recurrence
(32.7% vs. 50.8%; P= 0.021) and better median survival (83 months
vs. 31 months; P = 0.001)[19]. Similarly, Patients who completed
TMT ( chemoradiotherapy [CRT] and surgery)had the best local
control in a Single-Institution Experience conducted by [20]Sio TT
et al. 5-year local control was 82% for TMT, while 60% for CRT and
40% for PTMT patients who began treatment with trimodality intent
but did not undergo surgery groups (P<0.001).
Regarding if the role of adding surgery could be replaced by
escalated radiation dose in the CRT alone group. The current
National Comprehensive Cancer Network (NCCN) recommended
ranges for preoperative, postoperative and definitive radiation are
41.4 to 50.4 Gy, 45 to 50.4 Gy, and 50 to 50.4 Gy, respectively [21].
Despite the lack of significant evidence supporting radiation dose
escalation, we found that radiation doses exceeding 50.4 Gy were
used in 58.7% of patients in the CRT alone group in this retrospective
study due to the historical situation in China. However, we found no
survival benefit to a dose escalation of >50.4 Gy compared with those
receiving a 50 to 50.4 Gy radiation dose. A previous study on the use
of definitive CRT (the RTOG 8501 trial) showed that the 5-year OS
was 26% in patients receiving chemotherapy with radiotherapy (to a
total dose of 50 Gy) and 0% when radiotherapy (to total dose of 64
Gy) was used alone[3]. Owing to the low OS and high local failure
rate with definitive CRT, the Intergroup 0123 trial subsequently
randomized patients to receiving the same chemotherapy with either
50.4 Gy or 64.8 Gy of radiation [4]. However, the trial was stopped
early after an interim analysis showed the 2-year median survival (13
vs. 18.1 months) and locoregional failure rates (56% vs. 52%) were
not significantly different between the high-dose and standard-dose
arms. Similarly, our multivariate analysis indicated no differences in
OS and recurrence based on the radiation dose delivered in both the
trimodality and CRT alone groups. As no subsequent studies have
revealed a significant benefit to dose escalation exceeding 50.4 Gy in
CRT alone group, our study indicated that the role of surgery could
not be replaced by CRT alone, even with increased radiation dose for
locally advanced SCC of the esophagus.
The current study does have some limitations. For example, as this
was a retrospective study and only esophageal SCC patients in China
were recruited, we may have introduced selection bias and we lack a proper intent-to-treat analysis. Furthermore, while the incidence of esophageal adenocarcinoma is dramatically increasing in Western
countries, the results of our study should not be generalized to apply
to North American and European patients until a randomized study
including esophageal adenocarcinoma patients confirms our results.
Moreover, based on our study, its unclear what is the potential value of
additional locoregional therapy with surgery in patients with clinical
complete response to CRT.We also had no idea about the timing and
necessity of oesophagectomy in (all) patients. In the future, molecular
biology techniques probably may enable to improve prognostic
stratification, thereby allowing us to determine the types of patients
who benefit from surgical therapy and show improved OS [22-25].
In addition, we did not analyze whether patients received salvage
therapies, such as salvage esophagectomy, in the CRT alone group,
which may have affected survival outcomes. Further investigation of
the innovative multidisciplinary management (i.e., preoperative CRT
followed by salvage esophagectomy) for patients with locally advanced
esophageal cancer is warranted. Indeed, this approach is currently
being explored in Netherlands by investigators of the preSANO trial,
clinical response evaluation after neoadjuvantchemoradiotherapy in
esophageal cancer (NTR4834) [26].
In conclusion, adding surgery to CRT appears to have superior
local control, PFS and OS, with similar treatment-related mortality
for the treatment of patients with SCC of esophagus. The role of
surgery could not be replaced by CRT alone, even with increased
radiation dose.
Acknowledgement
This work was supported by the Zhejiang Provincial Program for the Cultivation of High-level Innovative Health Talents (to YapingXu). This paper has been accepted for oral presentation at the 15th World Congress of International Society for Diseases of the Esophagus, taking place on September 19-21, 2016 in Singapore (Abstract ID: 1942).
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90.
- Guo M, Zhao YD, Yang HJ, Yan XF. Analysis of clinicopathological characteristics for 5406 cases of esophageal neoplasm. Chin J Cancer Prev Treat. 2008;15:54-6.
- Herskovic A, Martz K, al-Sarraf M, Leichman L, Brindle J, Vaitkevicius V, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326:1593-8.
- Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167-74.
- Oppedijk V, van der Gaast A, van Lanschot JJ, van Hagen P, van Os R, van Rij CM, et al. Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the CROSS trials. J Clin Oncol. 2014;32:385-91.
- Christophe Mariette, Laetitia Dahan, Françoise Mornex, Emilie Maillard, Pascal-Alexandre Thomas, Bernard Meunier, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol. 2014;32:2416-22.
- Smalley SR, Benedetti JK, Haller DG, Hundahl SA, Estes NC, Ajani JA, et al. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol. 2012;30:2327-33.
- van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074-2084.
- Urschel JD, Vasan H. A meta-analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer. Am J Surg. 2003;185:538-43.
- Fiorica F, Di Bona D, Schepis F, Licata A, Shahied L, Venturi A, et al. Preoperative chemoradiotherapy for oesophageal cancer: a systematic review and meta-analysis. Gut. 2004;53:925-30.
- Bédard EL, Inculet RI, Malthaner RA, Brecevic E, Vincent M, Dar R. The role of surgery and postoperative chemoradiation therapy in patients with lymph node positive esophageal carcinoma. Cancer. 2001;91:2423-30.
- Rice TW, Adelstein DJ, Chidel MA, Rybicki LA, DeCamp MM, Murthy SC, et al. Benefit of postoperative adjuvant chemoradiotherapy in locoregionally advanced esophageal carcinoma. J Thorac Cardiovasc Surg. 2003;126:1590-96.
- Xu Y, Chen Q, Yu X, Zhou X, Zheng X, Mao W. Factors influencing the risk of recurrence in patients with esophageal carcinoma treated with surgery: A single institution analysis consisting of 1002 cases. Oncol Lett. 2013;5:185-90.
- Stahl M, Stuschke M, Lehmann N, Meyer HJ, Walz MK, Seeber S, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23:2310-7.
- Bedenne L, Michel P, Bouché O, Milan C, Mariette C, Conroy T, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25:1160-8.
- Neoadjuvant versus adjuvant therapy in treating resectable thoracic esophageal cancer. 2011.
- Shao MS, Wong AT, Schwartz D, Weiner JP, Schreiber D.Definitive or Preoperative Chemoradiation Therapy for Esophageal Cancer: Patterns of Care and Survival Outcomes. Ann Thorac Surg. 2016;101:2148-54.
- Vincent J, Mariette C, Pezet D, Huet E, Bonnetain F, Bouché O, et al. Early surgery for failure after chemoradiation in operable thoracic oesophageal cancer. Analysis of the non-randomised patients in FFCD 9102 phase III trial: Chemoradiation followed by surgery versus chemoradiation alone. Eur J Cancer. 2015;51:1683-93.
- Piessen G, Messager M, Mirabel X, Briez N, Robb WB, Adenis A, et al. Is there a role for surgery for patients with a completeclinical response after chemoradiation for esophageal cancer? An intention-to-treat case-control study. Ann Surg. 2013;258:793-9.
- Sio TT, Wilson ZC, Stauder MC,Bhatia Sumita,Martenson James A,Quevedo J Fernando, et al. Long-term Treatment Outcomes for Locally Advanced Esophageal Cancer: A Single-Institution Experience. Am J Clin Oncol. 2016;39:448-52.
- Ajani JA, D'Amico TA, Almhanna K, Bentrem DJ, Besh S, Chao J, et al. Esophageal and esophagogastric junction cancers, version 1.2015. J Natl Compr Canc Netw. 2015;13:194-227.
- Cheng-Che Tu, Po-Kuei Hsu, Ling-I Chien, Wan-Chen Liu, Chien-Sheng Huang,Chih-Cheng Hsieh, et al. Prognostic histological factors in patients with esophageal squamous cell carcinoma after preoperative chemoradiation followed by surgery. BMC Cancer. 2017;17:62.
- Chang-Juan Tao, Gang Lin, Ya-Ping Xu, Wei-Min Mao. Predicting the Response of Neoadjuvant Therapy for Patients with Esophageal Carcinoma: an In-depth Literature Review. J Cancer. 2015;6:1179-86.
- Hamai Y, Hihara J, Emi M, Furukawa T, Yamakita I, Kurokawa T, et al. Ability of Fluorine-18 Fluorodeoxyglucose Positron Emission Tomography to Predict Outcomes of Neoadjuvant Chemoradiotherapy Followed by Surgical Treatment for Esophageal Squamous Cell Carcinoma. Ann Thorac Surg. 2016; 102:1132-9.
- Shen LY, Wang H, Dong B, Yan WP, Lin Y, Shi Q, et al. Possible prediction of the response of esophageal squamous cell carcinoma to neoadjuvant chemotherapy based on gene expression profiling. Oncotarget. 2016;7:4531-41.
- Clinical response evaluation after neoadjuvant chemoradiotherapy in esophageal cancer. NTR4834. 2014.