Case Report
A Case Report of a Patient with HER2-Positive Metastatic Breast Cancer on Dialysis, Who Responded to Ado-Trastuzumab Emtansine
Elia Sais and Sonia Del Barco*
University Hospital Doctor Josep Trueta, The Catalan Institute of Oncology (ICO), Medical Oncology Service,
Girona, Spain
*Corresponding author: Sonia Del Barco Berrón, Servicio de Oncología Médica, Hospital Universitario Doctor Josep Trueta, Instituto Catalán de Oncología (ICO), Avda. França, s/n. 17007 Girona, Spain
Published: 20 Nov, 2017
Cite this article as: Sais E, Del Barco S. A Case Report
of a Patient with HER2-Positive
Metastatic Breast Cancer on Dialysis,
Who Responded to Ado-Trastuzumab
Emtansine. Ann Clin Case Rep. 2017;
2: 1471.
Abstract
Introduction: Ado-trastuzumab emtansine (T-DM1) is a human epidermal growth factor receptor
2 (HER2)-targeted drug that comprises trastuzumab, a stable linker, and the potent cytotoxic agent
derivative of maytansine (DM1). Once T DM1 binds to the HER2 receptor, it allows intracellular
drug delivery specifically to HER2-overexpressing cells, minimizing the exposure of the drug to
normal tissue.
Case: We report the case report of a 47-year old woman with HER2-positive metastatic breast
cancer (MBC) on dialysis, which was treated with T-DM1. The tolerance of T-DM1 in this patient
was predictable and good and the patient achieved a durable response.
Discussion and Conclusions: A previous exploratory analysis has shown that the pharmacokinetic
properties of T-DM1 are unaffected by age, race or renal function. However, there are no specific
trials for patients with severe renal impairment treated with T-DM1. Only one patient with these
characteristics has been treated before with T-DM1, although no dosage recommendation was
given.
Keywords: Antibodies; Antineoplastic agents; Breast neoplasms; Monoclonal; Renal dysfunction
Introduction
Breast cancers expressing human epidermal growth factor receptor 2 (HER2+) have higher rates of proliferation and a worse prognosis than non-HER2-expressing tumors without target therapy. This type of breast cancer represents about 15% of all breast cancer types. Despite adjuvant trastuzumab treatment, some of these patients will present a relapse. The arrival of new anti-HER2 therapies such as pertuzumab and ado-trastuzumab emtansine (T-DM1) have provided not only a benefit in terms of progression-free survival (PFS) but also an improvement in overall survival (OS) in patients with HER2+ metastatic breast cancer (MBC) [1]. This has led to the development of a guideline for the management of patients with HER2+ MBC by the American Society of Clinical Oncology (ASCO) [2]. This guideline addresses current knowledge and makes recommendations for the use of such treatments in first-line and beyond.
Case Presentation
A 47 year-old Caucasian woman presented to the district hospital in June 2012 with a history
of mammography screening with calcifications in the right breast. A breast biopsy reported a grade
III ductal carcinoma. She underwent a mastectomy and clearance of axillary nodes on the right side
in June 2012. Pathological analysis showed ductal carcinoma pT2 (5 cm) grade III, pN3a involving
9 of 14 nodes with capsular rupture, HER2+ and carcinoma in situ > 25%, hormone receptor
negative and 50% Ki-67 index, so an oncology referral was made. A contrast-enhanced computed
tomography (CT) scan also revealed multiple liver metastases and isolated bone metastases in dorsal
vertebrae.
She had been undergoing hemodialysis and immunosuppressive treatment since 1992 because
of kidney transplantation owing to bilateral renal atrophy while she was a teenager. New kidney
transplantation was performed in September 2011, as severe chronic glomerulopathy was observed
in the original transplanted kidney after a renal biopsy.
In July 2012, she received trastuzumab and docetaxel as first-line
treatment for the breast cancer until December 2012 every Thursday
on a three-week cycle because she was receiving hemodialysis on
Monday, Wednesday and Friday. She completed 6 cycles of treatment
with complete liver response and stable disease in bone. She continued
receiving trastuzumab until September 2013.
In September 2013, a CT scan showed disease progression in the
liver. She started treatment with capecitabine plus lapatinib as secondline
therapy. She showed gastrointestinal toxicity with diarrhea
grade 3 and, due to that, lapatinib was substituted by trastuzumab.
In January 2014, capecitabine was replaced with vinorelbine due to
the appearance of important gastrointestinal toxicity. In April 2014,
a new radiological examination showed complete response in liver
and stable disease in bone. In July 2014, the patient was referred to
our hospital in order to continue the treatment with vinorelbine plus
trastuzumab.
In August 2014, a CT scan revealed stable disease in the liver,
but new tumors in the peritoneum appeared (Figure 1). She started
T-DM1 in September 2014 as third-line treatment. A CT scan was
carried out in December 2014 after three months of treatment, which
revealed partial response in bone and complete response in peritoneal
carcinomatosis (Figure 2). Patient recived TDM1 for two years.
Finally in September 2016 she presented progression at SNC with
clinical deteroriation and she died three months later. During she was
receiving T-DM1 she had performance status 0, which allowed her to
perform normal life without any toxicity. The last CT performed in
September 2016 showed that the partial response in the bone and the
complete response in the peritoneum have been maintained (Figure
3).
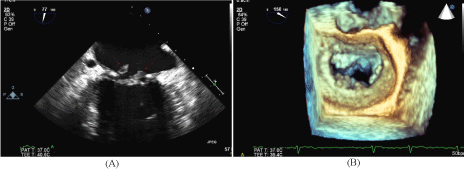
Figure 1
Figure 1
Trans-esophageal echocardiogram following stroke.
(A) 2-chamber view of the mitral valve in systole with vegetations attached to
the mitral annuloplasty ring.
(B) 3D TEE image of the mitral valve in diastole with multiple small vegetations
attached to the mitral annuloplasty ring.
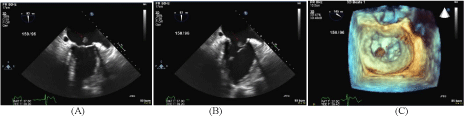
Figure 2
Figure 2
Follow-up trans-esophageal echocardiogram.
(A-B) 2-chamber view of the mitral valve in systole (A) and diastole (B) with a
large vegetation attached to the annuloplasty ring and posterior mitral valve
leaflet.
(C) 3D TEE image of the mitral valve in systole with a large vegetation
attached to the mitral annuloplasty ring.
Figure 2
Figure 2
Follow-up trans-esophageal echocardiogram.
(A-B) 2-chamber view of the mitral valve in systole (A) and diastole (B) with a
large vegetation attached to the annuloplasty ring and posterior mitral valve
leaflet.
(C) 3D TEE image of the mitral valve in systole with a large vegetation
attached to the mitral annuloplasty ring.
Discussion
T-DM1 is a HER2-targeted antibody-drug conjugate comprising
trastuzumab, a stable linker, and the potent cytotoxic agent derivative
of maytansine (DM1). Once T‑DM1 binds to the HER2 receptor, it
allows intracellular drug delivery specifically to HER2-overexpressing
cells, consequently improving the therapeutic index of the drug and
minimizing its exposure to normal tissue [3]. The EMILIA trial is a
phase III randomized, multicenter, international, open-label study
designed to compare the safety and efficacy of T-DM1 with capecitabine
plus lapatinib in HER2+ MBC patients who had previously received
trastuzumab and a taxane in the metastatic setting or developed
disease recurrence during or within 6 months of completing adjuvant
therapy [1]. In this trial, T-DM1 demonstrated improved median
PFS compared with capecitabine and lapatinib (9.6 vs. 6.4 months,
respectively; hazard ratio [HR] = 0.65; 95% confidence interval [CI]
0.55-0.77; p < 0.001). Median OS also favored T‑DM1 (30.9 vs. 25.1
months, respectively; HR = 0.68; 95% CI 0.55-0.85; p < 0.001). Overall
response rate and duration of response were also superior in patients
treated with T-DM1 [1]. These results led to the approval of T-DM1
in 2013 by the U.S. Food and Drug Administration (FDA) and the
European Medicines Agency (EMA) for the treatment as single agent
of patients with HER2+ MBC who previously received trastuzumab
and a taxane, separately or in combination.
The drug is given as an intravenous infusion every 3 weeks (21-day
cycle) until disease progression or unacceptable toxicity occurs. After
being administered, T-DM1 undergoes a process of deconjugation
and catabolism in cell lysosomes. DM1 is metabolized by CYP3A4
and CYP3A5 in liver microsomes. With a dose of 3.6 mg/kg, the
clearance is 12.9 ml/day/kg and the terminal half-live (t1/2) is 3.5 days
[4]. It has a linear pharmacokinetic process and preclinical trials show
that T-DM1 catabolites are eliminated by the biliary system with
minimal participation of the renal system [5].
An exploratory analysis has shown that the pharmacokinetic
properties of the drug are unaffected by age, race or renal function
[6]. In patients with low to moderate renal impairment, the
pharmacokinetic profile was similar to patients with normal
creatinine clearance. There are no specific trials for patients with
severe renal impairment (creatinine clearance between 15 ml/min-29
ml/min). Only one patient with these characteristics has been treated
with T-DM1, but there is no dosage recommendation. We presented
here the case report of a 47-year old patient with HER2+ MBC on
dialysis that was treated with T-DM1 outside a clinical trial. The
tolerance of T-DM1 in this patient was predictable and good and the
patient achieved a durable response.
References
- Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012; 367(19):1783-1791.
- Giordano SH, Temin S, Kirshner JJ, Chandarlapaty S, Crews JR, Davidson NE, et al. Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014; 32(19):2078-2099.
- Barok M, Joensuu H, Isola J. Trastuzumab emtansine: mechanisms of action and drug resistance. Breast Cancer Res. 2014; 16(2):209.
- Krop IE, Beeram M, Modi S, Jones SF, Holden SN, Yu W, et al. Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol. 2010; 28(16):2698-2704.
- Gupta M, Lorusso PM, Wang B, Yi JH, Burris HA 3rd, Beeram M, et al. Clinical implications of pathophysiological and demographic covariates on the population pharmacokinetics of trastuzumab emtansine, a HER2-targeted antibody-drug conjugate, in patients with HER2-positive metastatic breast cancer. J Clin Pharmacol. 2012; 52(5):691-703.
- Lu D, Girish S, Gao Y, Wang B, Yi JH, Guardino E, et al. Population pharmacokinetics of trastuzumab emtansine (T-DM1), a HER2-targeted antibody-drug conjugate, in patients with HER2-positive metastatic breast cancer: clinical implications of the effect of covariates. Cancer Chemother Pharmacol. 2014; 74(2):399-410.