Case Report
Rare 2p Gain with Complex Karyotype in a Symptomatic Patient with Small Lymphocytic Lymphoma/Chronic Lymphocytic Leukemia
Farhan Khan1, Rabia Zafar1, Lori Wilson2 and Tammey Naab1*
1Department of Pathology, Howard University, USA
2Department of Surgery, Howard University, USA
*Corresponding author: Tammey Naab, Department of Pathology, Howard University, 2041 Georgia Avenue, NW, Washington, DC, USA
Published: 13 Oct, 2017
Cite this article as: Khan F, Zafar R, Wilson L, Naab T.
Rare 2p Gain with Complex Karyotype
in a Symptomatic Patient with Small
Lymphocytic Lymphoma/Chronic
Lymphocytic Leukemia. Ann Clin Case
Rep. 2017; 2: 1448.
Abstract
Small lymphocytic lymphoma/chronic lymphocytic leukemia (SLL/CLL) is often an incidental finding in an elderly asymptomatic patient. We report a case of a 58 year old female, presenting with fever, night sweats and painless submandibular and supraclavicular lymphadenopathy. The left axillary lymph node excisional biopsy showed complete effacement of architecture by small mature lymphocytes. There were scattered pseudofollicles with pale nodular appearance containing large lymphocytes with open chromatin. The neoplastic cells were small with round nuclear contours, coarsely clumped chromatin and scant cytoplasm and they diffusely expressed CD20, CD5 and CD23 consistent with SLL/CLL. Ki-67 (20%) was increased outside of the proliferation centers. Flow cytometry revealed a clonal kappa population with coexpression of CD20, CD5 and CD23. Karyotyping revealed a complex abnormal karyotype with two neoplastic clones and several rearrangements, including a gain of 2p resulting from der (14) t (2;14), confirmed by concurrent FISH showing a MYCN (2p24) signal in the der (14). CLL with 2p gain is usually associated with unfavorable cytogenetic deletions (11q, 17p), unmutated status of immunoglobulin heavy chain (IGHV) and increased CD38. However, in our case, FISH showed loss of 13q and CD38 was low (5%), findings more often associated with a favorable prognosis.
Introduction
Chronic lymphocytic leukemia/small lymphocytic lymphoma (SLL/CLL) is a mature B cell malignancy. It is the most common leukemia in the elderly (>50 years) in the United States accounting for 22-30% of all leukaemia cases [1,2]. It is often diagnosed as an incidental finding in asymptomatic individuals who show peripheral blood lymphocytosis with small mature lymphocytes and characteristic smudge cells. It is the most common cause of generalized lymphadenopathy in the same age bracket [1,2]. Histologically, the lymph node architecture is effaced by mature B lymphocytes with condensed chromatin. The flow cytometry establishes the malignancy by detecting monoclonal B cell population. The diagnosis of SLL/CLL is made by the expression of CD20, CD5 and CD23 cell surface antigens by the neoplastic cells. The clinical courser is heterogeneous with most patients experiencing a stable clinical course and survival without treatment, while in a minority of cases, it has a progressive course [3-5]. The current clinical staging is not a reliable predictor of clinical behavior. Several biomarkers based on pregerminal versus postgerminal origin of SLL/CLL and recurrent cytogenetic abnormalities are used to predict the prognosis. The gain of short arm of chromosome 2 (2p) is a rare cytogenetic finding in SLL/CLL. In most studies, this finding is associated with adverse prognostic markers [6-8]. However, in our case, 2p gain was detected with conventional karyotyping, confirmed with subsequent fluorescent in situ hybridization (FISH) study and was associated with favorable prognostic markers.
Case Presentation
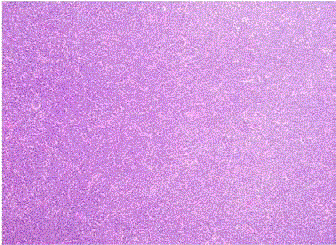
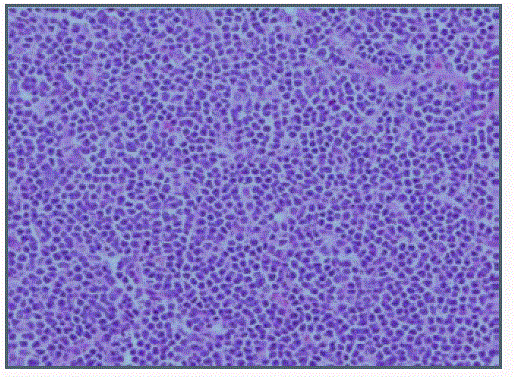
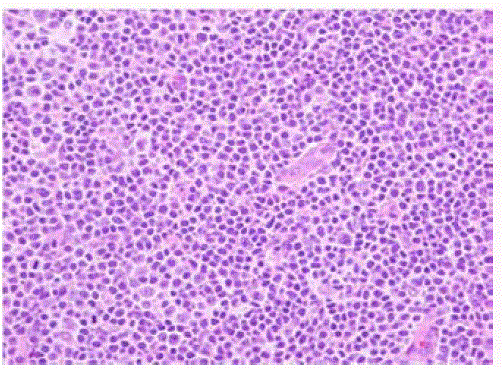
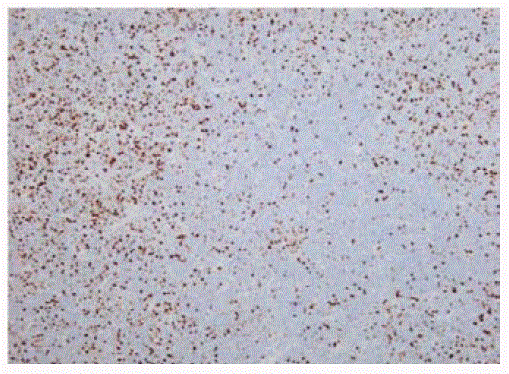
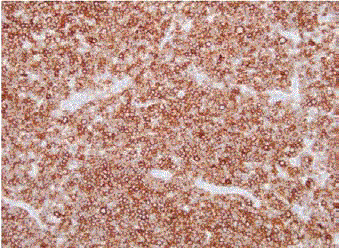
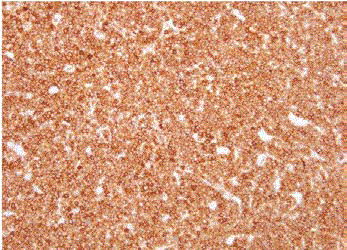
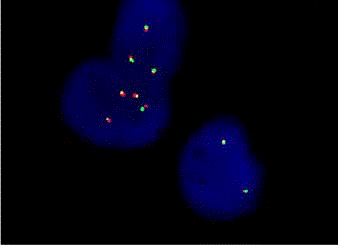
We report a case of a 58 year old female who presented with fever, night sweats and painless neck swelling. Complete blood count (CBC) showed white blood cell count of 10.5/microliters (3.2- 10.6/microliters) with 24% lymphocytes on differential count, red blood cell count of 4.12 (3.77- 5.28), hematocrit of 36.3 (34.0-46.6), hemoglobin of 11.9 g/dl (11.1-15.9 g/dl) and mean corpuscular volume (MCV) of 88 fl. CT scan showed bilateral submandibular and supraclavicular lymph node enlargement. Left axillary lymph node excision showed diffusely effaced architecture by a lymphoid population that extended through the capsule and involved perinodal adipose tissue. The infiltrate consisted of small round lymphocytes with condensed nuclear chromatin and scant cytoplasm (Figure 1,2). Admixed at regular intervals were proliferation centers which imparted a vague, pale nodular appearance. The proliferation centers were composed of larger lymphoid cells with more abundant cytoplasm, open nuclear chromatin and more conspicuous nucleoli (Figure 3). Ki-67 showed increased proliferation (20%) outside the proliferation centers (Figure 4) that raised some concern of early transformation to large B cell lymphoma. However, confluent sheets of large cells were not observed. The small mature lymphocytes weakly expressed CD20, CD5 and CD23 (Figures 5-7) and more strongly expressed BCL2. Cyclin D1, CD10 and BCL6 were not expressed by the neoplastic cells (Figure 8). Scattered reactive CD3 positive T lymphocytes were present. Flow cytometry revealed a clonal kappa positive, B cell population that co-expressed CD5, dim CD20 and CD23. CD38 was low (5%). These findings were consistent with SLL/ CLL, stage II. Karyotyping revealed a complex abnormal karyotype with two neoplastic clones and several rearrangements, including a gain of 2p resulting from der (14) t (2;14), confirmed by concurrent FISH showing a MYCN (2p24) signal in the der (14) (Figure 9). The FISH panel for SLL/CLL showed loss of 13q14 (Rb gene) with no abnormality in other FISH probes.
Figure 1
Figure 2
Figure 3
Figure 3
Prolymphocytes and paraimmunoblasts with pale cytoplasm and
mitosis in proliferation centers (x400).
Figure 4
Figure 5
Figure 6
Figure 7
Figure 8
Figure 9
Discussion
The terms SLL and CLL are used interchangeably, the former
presenting with a leukemic picture and the latter being limited to
disease in lymph nodes [9,10]. The most common clinical presentation
is an asymptomatic patient with an incidental finding of SLL/CLL
[11-15]. The peripheral blood smear shows lymphocytosis with
small mature lymphocytes and smudge cells (fragile degenerating
leukemic cells). SLL/CLL is the most common malignant cause of
generalized lymphadenopathy in an elderly population (>50 years)
with nonspecific symptoms of anorexia, fever, night sweats and
weight loss. In some cases, SLL/CLL may be associated with warm or,
less often, cold autoimmune hemolytic anemia [15-17].
Peripheral blood lymphocytosis in an elderly patient should raise
suspicion for SLL/CLL. The diagnosis of malignancy is established
with flow cytometry revealing a monoclonal B cell population.
In cases with lymphadenopathy, the lymph node architecture is
completely effaced by small mature lymphocytes having condensed
nuclear chromatin. Pseudofollicles have pale nodular appearance
due to the proliferation of large lymphocytes having open nuclear
chromatin and more abundant cytoplasm. SLL/CLL is a low grade
B-cell malignancy having a low proliferation index except the
pseudofollicles which may show increased Ki67. The expression of
CD20, CD5 and CD23 by the neoplastic cells either by flow cytometry
or by immunohistochemistry is consistent with SLL/CLL.
SLL/CLL is clinically heterogeneous with an indolent course
in most patients with good prognosis who may survive without
treatment for a considerable period of time [3-5]. In some patients,
SLL/CLL behaves as a progressive malignancy with decreased
survival. The current staging systems, Rai and Binet, identify patients
with advanced stage disease, who require treatment; but these staging
systems are not able to predict the clinical course [18]. The prognosis
of SLL/CLL depends upon pregerminal versus postgerminal origin of
malignancy [19]. Several molecular and cytogenetic markers are used
to classify SLL/CLL into distinct groups with prognostic significance.
The clinical course of SLL/CLL depends upon the somatic
hypermutation status of the immunoglobulin heavy chain variable
(IGHV) region. The somatic hypermutation of IGHV increases affinity
of the B cell receptor with antigen during an immune response. The
unmutated IGHV status (>95% homology to germline sequences) is
consistent with pregerminal center origin and poor prognosis. The
mutated IGHV status is consistent with postgerminal center origin
and predicts good outcome [20,21]. CD38, a cell surface antigen
and 70-kd zeta-chain T-cell receptor–associated protein kinase
(ZAP-70) are used as surrogate markers for IGHV mutation status
[22,23]. These biomarkers can be evaluated with flow cytometry or
immunohistochemistry. Increased CD38/ZAP-70 expression > 30%
is consistent with unmutated IGHV status, pregerminal center origin
and poor prognosis in SLL/CLL patients.
Recurrent cytogenetic abnormalities represent independent
markers for disease progression in SLL/CLL. Fluorescence in situ
hybridization (FISH) is the standard for detection of cytogenetic
abnormalities [24-26]. Loss of 13q14 (Rb gene) is established
as a favorable prognostic marker. Trisomy 12 is associated with
intermediate prognosis. Loss of 11q22 (p53 gene) and 17p13 (ATM
gene) carry poor prognosis [27,28]. SLL/CLL has low proliferative
index; therefore, limiting the role of conventional karyotyping in
evaluation of cytogenetic abnormalities.
Whole genome sequencing, comparative genomic hybridization
and single nucleotide pleomorphism (SNP) arrays have been used in
the evaluation of novel cytogenetic abnormalities in SLL/CLL from
a prognostic standpoint [29,30]. There are a few studies that have
identified gain of short arm of chromosome 2 (2p) as a rare novel
cytogenetic abnormality in SLL/CLL. These studies have linked the
2p gain with poor prognostic markers such as CD38/ZAP-70 high
expression and loss of 11q22 and 17p13 [6-8,31,32]. The rare 2p gain
is considered to be a marker of adverse prognosis in limited studies.
The gene transcription profiling has identified a few proto-oncogenes,
mapped to 2p, such as MYCN and REL. The dysregulation of these
oncogenes is suggested as a potential pathogenetic mechanism for
progression of SLL/CLL [33].
MicroRNAs are short, noncoding single-stranded RNA
molecules with length of approximately 22 nucleotides. MicroRNAs
may be useful as prognostic markers for B-cell malignancy because
their expression allows specific cell differentiation stages to be
identified [34,35]. The pathogenesis of CLL is better understood after
studying the expression of miRNAs. Loss of miR-15a/16-1 cluster was
identified from 13q14 locus in 2002. This cluster regulates BCL2 and
apoptosis, which explains BCL2 overexpression in CLL [36]. Some
studies showed that expression of miRNA-29 decreased as the disease
progressed, suggesting its role as a favorable prognostic marker
[37,38].
The 2p gain has been reported in at least 86 cases of CLL. Overall,
unfavorable cytogenetic deletions (11q22, 17p13) were more frequent
in 2p gain cases as well as unmutated IGHV status and increased
CD38/ZAP-70 expression [6-8,31,32]. However, in our case the 2p
gain was associated with favorable biomarkers, including low CD38
and 13q14 loss. This case study warrants additional studies to establish
the prognostic significance of 2p gain in SLL/CLL. Furthermore, even
though mitotic activity is very low in CLL, karyotyping should be
attempted because novel cytogenetic abnormalities can be identified,
which can be further evaluated by FISH [24-26].
Conclusion
The prognosis of SLL/CLL depends upon recurrent cytogenetic abnormalities and somatic hypermutation status of immunoglobulin heavy chain region. The rare 2p gain in SLL/CLL has been reported to occur with poor prognostic markers. In our case, 2p gain is associated with low CD38 and loss of 13q14, often considered favorable prognostic markers. This paradoxical association of 2p gain in our case warrants further studies on its role as a prognostic marker in SLL/CLL.
References
- Redaelli A, Laskin BL, Stephens JM, Botteman MF, Pashos CL. The clinical and epidemiological burden of chronic lymphocytic leukaemia. Eur J Cancer Care (Engl). 2004; 13: 279-287.
- Stephens JM, Gramegna P, Laskin B, Botteman MF, Pashos CL. Chronic lymphocytic leukemia: economic burden and quality of life: literature review. Am J Ther. 2005; 12: 460-466.
- Mato A, Nabhan C, Kay NE, Weiss MA, Lamanna N, Kipps TJ, et al. Real-world clinical experience in the Connect® chronic lymphocytic leukaemia registry: a prospective cohort study of 1494 patients across 199 US centres. Br J Haematol. 2016; 175: 892-903.
- Pflug N, Bahlo J, Shanafelt TD, Eichhorst BF, Bergmann MA, Elter T, et al. Development of a comprehensive prognostic index for patients with chronic lymphocytic leukemia. Blood. 2014; 124: 49-62.
- Davis TA, Czerwinski DK, Levy R. Therapy of B cell lymphoma with anti-CD20 antibodies can result in the loss of CD20 antigen expression. Clin Cancer Res. 1999; 5: 611-615.
- Haferlach C, Jeromin S, Stengel A, Meggendorfer M, Zenger M, Kern W, et al. In: Chronic Lymphocytic Leukemia the Gain of the Short Arm of Chromosome 2 Is Highly Associated with an Unmutated IGHV status, 11q/ATM Deletion, SF3B1 Mutation and a Complex Karyotype.
- Fabris S, Mosca L, Cutrona G, Lionetti M, Agnelli L, Ciceri G, et al. Chromosome 2p gain in monoclonal B-cell lymphocytosis and in early stage chronic lymphocytic leukemia. Am J Hematol. 2013; 88: 24-31.
- Van Bockstaele F, Verhasselt B, Philippé J. Prognostic markers in chronic lymphocytic leukemia: a comprehensive review. Blood Rev. 2009; 23: 25-47.
- Santos FP, O'Brien S. Small lymphocytic lymphoma and chronic lymphocytic leukemia: are they the same disease?. Cancer J. 2012; 18: 396-403.
- Pangalis GA, Angelopoulou MK, Vassilakopoulos TP, Siakantaris MP, Kittas C. B-chronic lymphocytic leukemia, small lymphocytic lymphoma, and lymphoplasmacytic lymphoma, including Waldenström's macroglobulinemia: a clinical, morphologic, and biologic spectrum of similar disorders. Semin Hematol. 1999; 36: 104-114.
- Strati P, Shanafelt T. Monoclonal B-cell lymphocytosis and early-stage chronic lymphocytic leukemia: diagnosis, natural history, and risk stratification. Blood. 2015; 126: 454-462.
- Shanafelt TD, Kay NE, Rabe KG, Call TG, Zent CS, Maddocks K, et al. Brief report: natural history of individuals with clinically recognized monoclonal B-cell lymphocytosis compared with patients with chronic lymphocytic leukemia. J Clin Oncol. 2009; 27: 3959-3963.
- Parikh SA, Kay NE, Shanafelt TD. Monoclonal B-cell lymphocytosis: update on diagnosis, clinical outcome, and counseling. Clin Adv Hematol Oncol. 2013; 11: 720-729.
- Mowery YM, Lanasa MC. Clinical aspects of monoclonal B-cell lymphocytosis. Cancer Control. 2012; 19: 8-17.
- Kolyvanos Naumann U, Kaser L, Vetter W. Chronic lymphocytic leukemia (CLL). Chief symptoms: frequently asymptomatic, lymphadenopathy, splenomegaly. Praxis. 2003; 92: 2131-2135.
- Hollema H, Visser L, Poppema S. Small lymphocytic lymphomas with predominant splenomegaly: a comparison of immunophenotypes with cases of predominant lymphadenopathy. Mod Pathol. 1991; 4: 712-717.
- Motyckova G, Steensma DP. Why does my patient have lymphadenopathy or splenomegaly?. Hematol Oncol Clin North Am. 2012; 26: 395-408.
- Parikh SA, Strati P, Tsang M, West CP, Shanafelt TD. Should IGHV status and FISH testing be performed in all CLL patients at diagnosis? A systematic review and meta-analysis. Blood. 2016; 127: 1752-1760.
- Stevenson F, Caligaris-Cappio F. Chronic lymphocytic leukemia: revelations from the B-cell receptor. Blood. 2004; 103: 4389-4395.
- Blachly JS, Ruppert AS, Zhao W, Long S, Flynn J, Flinn, et al. Immunoglobulin transcript sequence and somatic hypermutation computation from unselected RNA-seq reads in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2015; 112: 4322-4327.
- Baliakas P, Agathangelidis A, Hadzidimitriou A, Sutton LA, Minga E, Tsanousa A, et al. Not all IGHV3-21 chronic lymphocytic leukemias are equal: prognostic considerations. Blood. 2015; 125: 856-859.
- Gogia A, Sharma A1, Raina V, Kumar L, Gupta R, Kumar R. Prevalence of ZAP-70 and CD 38 in Indian chronic lymphocytic leukemia patients. Indian J Cancer. 2013; 50: 333-336.
- Gachard N, Salviat A, Boutet C, Arnoulet C, Durrieu F, Lenormand B, et al. Multicenter study of ZAP-70 expression in patients with B-cell chronic lymphocytic leukemia using an optimized flow cytometry method. Haematologica. 2008; 93: 215-223.
- Eid OM, Eid MM, Kayed HF. Mahmoud WM, Mousafa SS, Ismail MM, Abdeen DM. Detection of cytogenetics abnormalities in chronic lymphocytic leukemia using FISH technique and their prognostic impact. Gulf J Oncolog. 2014; 1: 68-75.
- Xu W, Li JY, Li L, Yu H, Shen QD, Fan L, et al. Fluorescent in situ hybridization with a panel of probes detects molecular cytogenetic abnormalities in patients with chronic lymphocytic leukemia. Zhonghua Yi Xue Za Zhi. 2008; 88: 2537-2540.
- Xu W, Li JY, Pan JL, Qiu HR, Shen YF, Li L, et al. Interphase fluorescence in situ hybridization detection of cytogenetic abnormalities in B-cell chronic lymphocytic leukemia. Int J Hematol. 2007; 85: 430-436.
- Dickinson JD, Gilmore J, Iqbal J, Sanger W, Lynch JC, Chan J, et al. 11q22.3 deletion in B-chronic lymphocytic leukemia is specifically associated with bulky lymphadenopathy and ZAP-70 expression but not reduced expression of adhesion/cell surface receptor molecules. Leuk Lymphoma. 2006; 47: 231-244.
- Greipp PT, Smoley SA, Viswanatha DS, Frederick LS, Rabe KG, Sharma RG, et al. Patients with chronic lymphocytic leukaemia and clonal deletion of both 17p13.1 and 11q22.3 have a very poor prognosis. Br J Haematol. 2013; 163: 326-333.
- Zhao Z, Goldin L, Liu S. Evolution of multiple cell clones over a 29-year period of a CLL patient. Nat Commun. 2016; 7: 13765.
- Ouillette P, Saiya-Cork K, Seymour E, Li C, Shedden K, Malek SN. Clonal evolution, genomic drivers, and effects of therapy in chronic lymphocytic leukemia. Clin Cancer Res. 2013; 19: 2893-2904.
- Gachard N, Salviat A, Boutet C, Arnoulet C, Durrieu F, Lenormand B, et al. Multicenter study of ZAP-70 expression in patients with B-cell chronic lymphocytic leukemia using an optimized flow cytometry method. Haematologica. 2008; 93: 215-223.
- Dickinson JD, Gilmore J, Iqbal J, Sanger W, Lynch JC, Chan J, et al. 11q22.3 deletion in B-chronic lymphocytic leukemia is specifically associated with bulky lymphadenopathy and ZAP-70 expression but not reduced expression of adhesion/cell surface receptor molecules. Leuk Lymphoma. 2006; 47: 231-244.
- Rinaldi A, Mian M, Kwee, Rossi, Deambrogi C, Mensah AA, et al. Genome-wide DNA profiling better defines the prognosis of chronic lymphocytic leukaemia. Br J Haematol. 2011; 154: 590-599.
- Calin GA, Pekarsky Y, Croce CM. The role of microRNA and other non-coding RNA in the pathogenesis of chronic lymphocytic leukemia. Best Pract Res Clin Haematol. 2007; 20: 425-437.
- Calin GA, Croce CM. Genomics of chronic lymphocytic leukemia microRNAs as new players with clinical significance. Semin Oncol. 2006; 33: 167-173.
- Calin G, Dumitru C, Shimizu M, Bichi R, Zupo S, Noch E, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2002; 99: 15524-15529.
- Santanam U, Zanesi N, Efanov A, Costinean S, Palamarchuk A, Hagan JP, et al. Chronic lymphocytic leukemia modeled in mouse by targeted miR-29 expression. Proc Natl Acad Sci U S A. 2010; 107: 12210-12215.
- Pekarsky Y, Croce CM. Is miR-29 an oncogene or tumor suppressor in CLL?. Oncotarget. 2010;1: 224-227.