Case Report
Glands in Pleura-Not Always a Malignancy
Rabia Zafar, Farhan Khan and Tammey Naab*
Department of Pathology, Howard University, USA
*Corresponding author: Tammey Naab, Department of Pathology, Howard University, 2041 Georgia Avenue, NW, Washington, DC, USA
Published: 13 Oct, 2017
Cite this article as: Zafar R, Khan F, Naab T. Glands in
Pleura-Not Always a Malignancy. Ann
Clin Case Rep. 2017; 2: 1445.
Abstract
Normal visceral and parietal pleuron contain collagen, elastin fibers, lymphatics, small nerves, and blood vessels and is lined by a monolayer of mesothelium. The presence of epithelial cells in pleural tissue is a concern for a malignant process. We report a case of a 76 year old African American female, presenting with complaint of bleeding per rectum. The computed topography (CT) scan of the abdomen and pelvis showed rectosigmoid wall thickening, a large fluid attenuated mass within the pelvis and a right middle lobe lung nodule. Image guided drainage of the pelvic mass revealed neutrophils and gram negative rods but no evidence of malignancy. Colonoscopy with biopsy of the sigmoid mass revealed invasive moderately differentiated colonic adenocarcinoma. Fine needle aspiration (FNA) of the lung nodule showed clusters of malignant cells with rare acinar configuration and intracytoplasmic mucin. The tumor cells were positive for TTF-1 and CK7 and negative for CK20; these findings were consistent with primary lung adenocarcinoma. The core needle biopsy of the lung nodule did not show evidence of carcinoma. A well-defined cluster of benign epithelial cells with glandular configuration and a separate gland lined by ciliated columnar epithelium were identified in pleura. These benign glands expressed CK7 and TTF-1. The benign mesothelial cells lining the pleura were positive for CK5 and CK7. This case study highlights the importance of recognizing this benign condition, especially in patients with co-existing malignancy in order to avoid a false positive diagnosis of malignancy involving pleura.
Keywords: Pleura; Epithelial inclusion; Mesothelium; Lung adenocarcinoma
Introduction
The normal constituent of pleura is fibrous connective tissue composed of mainly elastic and collagen fibers, along with lymphatic, nerves and blood vessels. The lining of this connective tissue is single layer of mesothelium. A small amount of serous fluid is normally present between the parietal and visceral layers of pleura. Epithelial cells do not constitute normal component of pleura; thus, their presence raises the possibility of malignancy. We report a case of 76 year old African American female who was diagnosed with synchronous primary colon adenocarcinoma and primary adenocarcinoma of the right lung. The benign epithelial inclusions lined by columnar epithelium with glandular configuration were identified in the pleura during biopsy of the right lung nodule. This case study highlights that the finding of epithelial cells within the pleura does not always indicate metastasis, extension of primary lung carcinoma, primary mesothelioma, or endometriosis.
Case Presentation
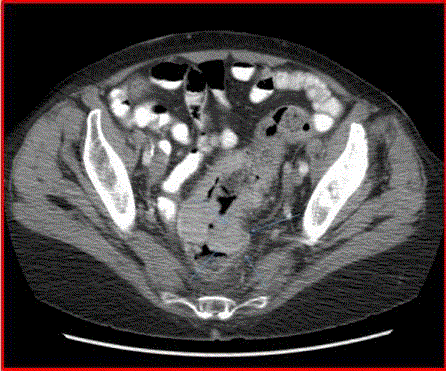
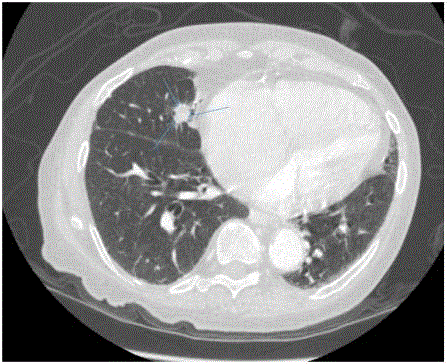
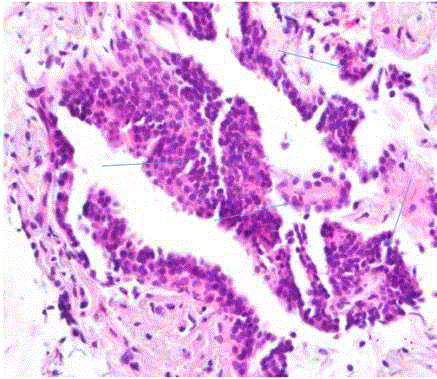
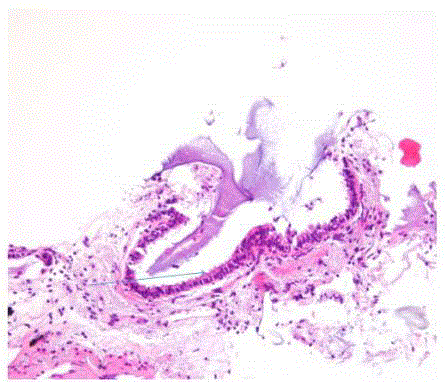
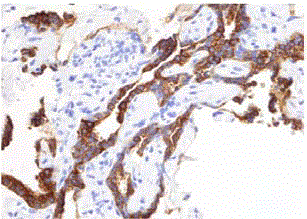
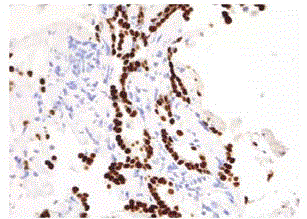
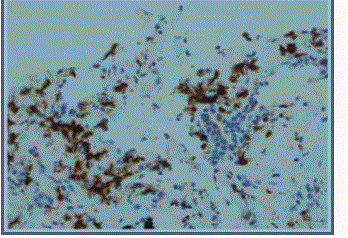
We report a case of a 76 year old African American female, presenting with rectal bleeding. The computed topography (CT) scan of the abdomen and pelvis showed rectosigmoid wall thickening, a large fluid attenuated pelvic mass, and a right middle lobe lung nodule (Figure 1,2). Image guided drainage of the pelvic mass revealed neutrophils and gram negative rods but no evidence of malignancy. The patient was started on broad spectrum antibiotics. Colonoscopy with sigmoid mass biopsy revealed invasive moderately differentiated colonic adenocarcinoma. CT guided fine needle aspiration (FNA) and biopsy of the lung nodule was performed. FNA showed clusters of malignant cells with rare acinar configuration and intracytoplasmic mucin. The tumor cells were positive for TTF-1 and CK7 and negative for CK20, consistent with primary lung adenocarcinoma. The biopsy of the lung nodule did not show carcinoma in multiple levels. A well-defined cluster of benign epithelial cells with glandular configuration and a separate gland lined by ciliated columnar epithelium were identified in the pleura (Figure 3,4); these bland-appearing cells expressed CK7 and TTF-1 (Figure 5,6). The benign mesothelial cells lining the pleura were positive for CK5 (Figure 7) and CK7.
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
Figure 6
Figure 7
Discussion
Histological finding of glands within pleura is concerning for the
presence of metastasis, advanced primary lung adenocarcinoma, or
primary mesothelioma since pleura normally consists of connective
tissue with interspersed nerves, lymphatics and blood vessels and a
lining of mesothelial cells. The most common type of malignancy
involving the pleura is adenocarcinoma characterized by the presence
of malignant glands in the pleura. It is often accompanied with malignant pleural effusion and dyspnea. The most common causes
of malignant pleural effusions include primary lung adenocarcinoma
(35%), metastasis from breast carcinoma in females (23%) and
lymphoma (10%) [1-3]. The metastasis from gastrointestinal tract
and gynaecological tract are rare in pleura. Although benign causes
of glands in pleura are rare, endometriosis (endometrial implants in
pleura) is the most significant cause [4,5].
Epithelial and/or nonepithelial inclusions are most commonly
reported in lymph nodes [6-12]. Patients with endometriosis may
have endometrial glands in pelvic and para aortic lymph nodes
[13]. Ectopic glands are relatively rare above the diaphragm, most
commonly found in cervical and axillary lymph nodes. Ectopic
glandular breast tissue and cystic structures lined by squamous
epithelium are found in axillary lymph nodes [14,15]. Ectopic
thyroid tissueis found occasionally in cervical lymph nodes. Among
nonepithelial implants, the most common are mesothelial inclusions
in mediastinal lymph nodes [16,17] peritoneum [17,18] as well
as below the diaphragm in abdominal lymph nodes. Peritoneal
endometriosis and surface epithelial inclusions of ovary are some
examples of extranodal glandular inclusions.
There are several theories for the explanation of ectopic glands.
Among lymph node implants, one theory proposes maldevelopment
at the embryonic level when glandular cells were entrapped within lymph nodes and survived. The other possibility may be the
mobilization of glands from their original site via lymphatics to
the lymph nodes. The cause of mobilization may be inflammation
or infection at the original site as seen in endometriosis where the
inflammation due to menstruation dislodges the glands and they are
transported via lymphatics to regional and/or distant lymph nodes.
These two theories also hold true for ectopic mesothelial inclusions
[19,20]. Epithelial implants on mesothelium can also be explained by
metaplasia. Embryonic coelomic and/or subcoelomic mesenchyme
undergoes metaplastic change to mesothelium. Chronic inflammation
may play a role in it. Regardless of the cause, these implants usually
raise the suspicion for malignancy but the benign causes of these
ectopic glands must be ruled out. This is especially important if
the patient has a history of malignancy as lung is a common site of
metastasis or coexisting/history of primary lung adenocarcinoma.
We expect to see elastic and collagen fibres in pleura, along
with nerves, blood vesselsand lymphatics, lined by a single layer of
mesothelium. Diagnosis of malignant glands in pleura makes lung
carcinoma inoperable and in the case of distant metastasis indicates
stage IV. This case study suggests that the false positive diagnosis of
pleural malignancy should be avoided and presence of benign glands
in pleura must be identified based on morphology.
Conclusion
There is no specific immunostain to distinguish benign glands from primary lung adenocarcinoma in pleura. Careful evaluation of morphology is the key to establish the diagnosis. This case study highlights the importance of considering the presence of benign glands in pleura in order to avoid the false positive diagnosis of advanced malignancy or stage IV metastatic disease from nonpulmonary cancers. This might be particularly challenging in patients with synchronous lung and non-pulmonary carcinoma.
References
- Johnston WW. The malignant pleural effusion: a review of cytopathologic diagnoses of 584 specimens from 472 consecutive patients. Cancer. 1985; 56: 905-909.
- Celikoglu F, Teirstein AS, Krellenstein DJ, Strauchen JA. Pleural effusion in non-Hodgkin's lymphoma. Chest. 1992; 101: 1357-1360.
- Aydin Y, Turkyilmaz A, Intepe YS, Eroglu A. Malignant Pleural Effusions: Appropriate Treatment Approaches. Eurasian J Med. 2009; 41: 186-193.
- Moffatt SD, Mitchell JD. Massive pleural endometriosis. Eur J Cardiothorac Surg. 2002; 22: 321-323.
- Dhanaworavibul K1, Hanprasertpong J, Cheewadhanaraks S, Buhachat R. Bilateral pleural endometriosis. J Obstet Gynaecol Res. 2006; 32: 86-89.
- Chen KT. Benign glandular inclusions of the peritoneum and periaortic lymph nodes. Diagn Gynecol Obstet. 1981; 3: 265-268.
- Fisher C, Hill S, Millis R. Benign lymph node inclusions mimicking metastatic carcinoma. J Clin Pathol. 1994; 47: 245-247.
- Resetkova E, Hoda S, Clarke J, Rosen P. Benign heterotopic epithelial inclusions in axillary lymph nodes. Histological and immunohistochemical patterns. Arch Pathol Lab Med. 2003; 127: e25-7.
- Garret R, Ada AEW. Epithelial inclusion cysts in an axillary lymph node: report of a case simulating metastatic adenocarcinoma. Cancer. 1957; 10: 173-178.
- Holdsworth PJ, Hopkinson JM, Leveson SH. Benign axillary epithelial lymph node inclusions-a histological pitfall. Histopathology. 1988; 13: 226-228.
- Parkash V, Vidwans M, Carter D. Benign mesothelial cells in mediastinal lymph nodes. Am J SurgPathol. 1999; 23: 1264-1269.
- Fellegara G, Carcangio ML, Rosai J. Benign epithelial inclusions in axillary lymph nodes: report of 18 cases and review of the literature. Am J SurgPathol. 2011; 35: 1123-1133.
- Burnett RA, Millan D. Decidual change in pelvic lymph nodes: a source of possible diagnostic error. Histopathology. 1986; 10: 1089-1092.
- Maiorano E, Mazzarol GM, Pruneri G, Mastropasqua MG, Zurrida S, Orvieto E, et al. Ectopic breast tissue as a possible cause of false positive axillary sentinel lymph node biopsies. Am J SurgPathol. 2003; 27: 513-518.
- Layfield LJ, Mooney E. Heterotopic Epithelium in an Intramammary Lymph Node. Breast J. 2000; 6: 63-67.
- Brooks JS, LiVolsi VA, Pietra GG. Mesothelial cell inclusions in mediastinal lymph nodes mimicking metastatic carcinoma. Am J Clin Pathol. 1990; 93: 741-748.
- Sethna K, Mohamed F, Marchettini P, Elias D, Sugarbaker PH. Peritoneal cystic mesothelioma: a case series. Tumori. 2003; 89: 31-35.
- Cohn DE, Folpe AL, Gown AM, Goff BA. Mesothelial pelvic lymph node inclusions mimicking metastatic thyroid carcinoma. Gynecol Oncol. 1998; 68: 210-213.
- Carney E, Cimino-Mathews A, Argani C, Kronz J, Vang R, Argani P. A Subset of Nondescript Axillary Lymph Node Inclusions Have the Immunophenotype of Endosalpingiosis. Am J SurgPathol. 2014; 38: 1612-1617.
- Perrone T. Embolization of benign colonic glands to mesenteric lymph nodes. Am J Surg Pathol. 1985; 9: 538-541.