Case Report
Levonorgestrel Intrauterine Device and Preservation of Fertility in Endometrial Cancer Grade I: Case Report and Literature Review
Jordi Rabasa Antonijuan1*, Gemma Escribano1, Ana María Alcalde Domínguez2, Rafael Sanchez Borrego1 and Jaume Pahissa Fabregas1
1Diatrecnon SLP, Teknon Medical Center, Spain
2Sacred Heart University Hospital - Quirónsalud Group, Barcelona, Spain
*Corresponding author: Jordi Rabasa Antonijuan, Diatrecnon SLP, Teknon Medical Center, Carrer de Vilana, 12, 08022 Barcelona, Spain
Published: 07 Oct, 2017
Cite this article as: Antonijuan JR, Escribano G,
Domínguez AMA, Borrego RS,
Fabregas JP. Levonorgestrel
Intrauterine Device and Preservation of
Fertility in Endometrial Cancer Grade
I: Case Report and Literature Review.
Ann Clin Case Rep. 2017; 2: 1440.
Abstract
Endometrial cancer is the most frequent gynecologic cancer. Although it mainly occurs in postmenopausal women, it can hit younger patients as well. Hysterectomy is considered the standard treatment and it could represent a problem for those young women who desire to preserve fertility. A conservative management can be offered to these patients when the tumor is well differentiated and advanced stage is excluded. Several studies are available in literature about fertility-sparing treatment in young women. Progestin treatment, seem to be the most validated conservative management. We report the case of a 43 years old patient, nulliparous, diagnosed by directed biopsy guided by hysteroscopy of grade (G)1 endometrial cancer stage IA. After the conservative treatment levonorgestrel-releasing intrauterine device (LNG-IUD) (Mirena, Bayer HealthCare Pharmaceuticals Inc.; 52 mg), the patient entered in complete remission. She conceived by In vitro Fertilization (IVF) treatment and delivered at 31 weeks multiple gestation by cesarean section for obstetric indication. After evaluation the case, total laparoscopic hysterectomy with bilateral salpingectomy was performed five months after delivery.
Keywords: Endometrial cancer; Fertility-sparing therapy; Progestin; Levonorgestrel-releasing intrauterine device; Reproduction; Outcomes
Case Presentation
We present a case of a nulliparous patient of 43-year-old with a 12 months history of abnormal
bleedings was referred on July 2013. Menarche was at age 12 and menses were irregular. The patient
didn't suffer dysmenorrhea, nor other gynecologic disease.
Her body mass index was 30, she was not affected by diabetes mellitus, and her family history
was negative for ovarian, uterine and colonic cancer.
After several months of irregular bleeding, a transvaginal ultrasound was carried out and
showed a normal-sized, anteverted uterus with a thickened endometrium and increased vascularity
within the endometrium. Ambulatory endometrial biopsy obtained with Cornier’spipelle showed
endometrioid adenocarcinoma grade I. The immunohistochemical study revealed positivity
for estrogen (85%) and progesterone (60%) receptors. Hysteroscopy (Betocchi® hysteroscope
5 mm, Karl Storz) revealed a friable and sessile mass inside the uterine cavity with atypical
vascularization. Isthmus and cervix were unaffected. Magnetic resonance imaging (MRI) discarded
myometrial infiltration. Lymph nodes were not involved by MRI. The patient desired future
fertility and conservative management of her disease, and therefore underwent levonorgestrelreleasing
IUD placement. Patients with endometrium-confined, well-differentiated, endometrioid
adenocarcinoma are the proper candidates for this treatment [1]. Endometrial sampling obtained
by betocchi hysteroscope in out patient basis, using semirigid grippers three months following IUDLNG
placement, showed complete regression of the adenocarcinoma. The patient has subsequently
undergone endometrial sampling, using Betocchi hysteroscope (5 mm) and semirigid grippers, every
three months. All specimens have been negative. Two follow-up MRI scans have been performed
with no other evidence of intrauterine or metastatic disease. The patient remained without evidence
of disease 10 months after initial IUD-LNG placement.
Thus, LNG-IUS was removed at 11 months after diagnosis, and it
was decided, given the age of the patient, an ovodonation to achieve
the best index of gestation. This is in line with the recently published
European Society of Gynecological Oncology (ESGO) guidelines [2],
stating that patients with previous infertility or risk factor of infertility
should be referred and encouraged to consider ART. Actually,
evidence shows that ART is a safe and effective procedure in this
setting [3]. The preparation consisted in an inhibition with triptorelin
depot prior to and subsequent endometrial estradiol transdermal
preparation to have an endometrial thickness greater than 8 mm but
with blood estrogen levels no greaterthan 200 pg /ml. Afterwards,
endometrial maturation was made with micronized progesterone in
high doses (800 mg / 24h).
Two embryos were transferred. Twelve and 14 days after embryo
transfer, serial blood β-hCG assays showed a biochemical pregnancy.
At 5 weeks from embryo transfer, a single viable pregnancy was
detected at ultrasound. At 27th week the patient was admitted due
to the threat of preterm labor. At 31 weeks + 4 days of gestation,
two healthy male babies delivered by cesarean section by obstetric
indication.
During cesarean section, multiple decidual biopsies for
intraoperative frozen sections were performed (resulting negative),
the abdomen was carefully inspected showing no macroscopic
cancer spread and random peritoneal biopsies were also obtained.
All definitive histological examinations of decidual, peritoneal, and
placental biopsies resulted negative.
A total laparoscopic hysterectomy with bilateral salpingooophorectomy
was performed four months after cesarean section.
Histological examination of the uterus showed no areas of complex
atypical hyperplasia or endometrial cancer. After 6 months of followup,
the patient is free of disease.
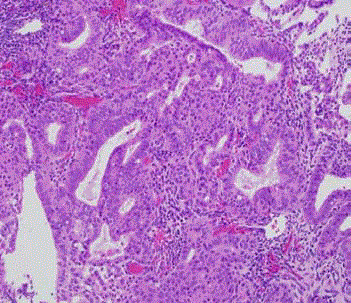
Figure 1
Figure 1
Well-differentiated endometrial adenocarcinoma without lymphatic
vascular invasion. The arrow indicates the most representative area of
endometrial cancer.
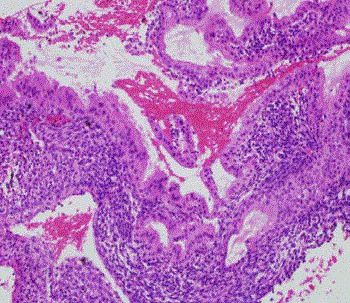
Figure 2
Figure 2
Post-treatment sample. Scarce glands and atrophic. The arrow
indicates the most representative area of atrophic glands after treatment with
progestins.
Discussion
Endometrial cancer is the most common of the gynecologic
malignancies [4]. Although it is primarily a disease of postmenopausal
women, 25% are premenopausal and 3% - 5% are under age 40 [5].
Endometrioid adenocarcinoma, the most common type, is typically
an estrogen driven disease affecting obese women.
Risk factors
Obesity and any condition that cause hyperestrogenic state are
the main risk factors for endometrial cancer in young women [6].
A literature review, about fertility sparing treatment in young
women with endometrial cancer, reported that the majority of
patients had a history of anovulation, ovarian dysfunction, nulliparity
and obesity [7].
Among young patients, thin women seem to have a more advanced
stage compared to those who are obese. Duska [8], demonstrated
that patients with BMI lower than 25 were more likely to have
advanced disease and high-risk histology (uterine serous papillary,
clear cell) compared with those women with a BMI over 25. Lee et
al. [9] reported that young women who were obese, hypertensive and
diabetic, tended to have well-differentiated tumours more frequently
compared to whose patients without metabolic disease. A comparison
of the disease in young and old-women is mandatory. The differences
between two groups were exposed in the study of Setiawan et al. [10]
and Colombo et al. [11].
The distribution of stage from I to IV and the histological type
were similar, but grade I endometrial cancer seem to occur more
frequently in young patients with less aggressive behaviour.
In this study, they demonstrated a higher prevalence of synchronic
ovarian malignancies in the younger group. The higher prevalence of
synchronous ovarian cancer in younger women is demonstrated in
several studies. Walsh [12] reported a rate of 25%, while Gitsch [13]
reported a rate of 29%.
In addition to this, young women with endometrial cancer
should always be carefully consulted about the need for a genetic
test for detection of Lynch syndrome, depending either on their
family history of cancer or depending of testing for mismatch
repair protein expression using microsatellite instability testing and
immunohistochemistry analysis. This will alert and identify patients
with Lynch syndrome who need a very close monitoring and tailored
consultation about their further follow-up and management. It
is debatable whether a patient with Lynch syndrome should be
candidate to conservative management.
Extensive counselling of the patient is the integral part of the
management. The detailed information about all aspects and risk
of conservative treatment has to be provided and informed consent
obtained, before initiation of the treatment.
Patient selection
The selection of endometrial cancer patients for whom fertilitysparing
treatment is appropriate is the aim to achieve the best
outcomes. All relevant studies recommended for patients with
early-stage, well-differentiated, endometrioid type endometrial
adenocarcinoma with no evidence of myometrial invasion or
extrauterine spread. According to the International Federation of
Obstetrics and Gynecology (FIGO) staging system, stage IA (confined
to endometrium), grade 1 endometrioid adenocarcinoma cases are
eligible for fertility-sparing treatment.
Well-differentiated tumours have a very low risk of myometrial
invasion and extrauterine spread (lymph node, ovarian or peritoneal
metastasis). In addition to this, well-differentiated tumour cells
express more progesterone receptors and therefore respond to
progesterone therapy [14].
In the other hand, the absence of myometrial invasion is also
important clinical aspect of endometrioid adenocarcinoma, because
implies a very low risk of extrauterine disease.
Navarria et al. [15] reported the estimated number of patients
who may need fertility – sparing treatment is a based population as a
rate of 0.3 in 100.000 women of these criteria.
However, because the incidence of young women with
endometrial cancer is increasing and the number of women who
want to delay having children, the future need for fertility – sparing
treatment will increase.
Management
Although hysterectomy represents the standard treatment for
endometrial cancer, it is often not accepted when the patient is young
and desires a pregnancy in the future.
In these cases, a fertility-sparing treatment could be offered as an
alternative option to accurately selected patients. Hormonal therapy
alone or combined with endometrial ablation by hysteroscopy are
identified in literature as the most used and effective conservative
treatments. However, patients must be informed that data about
medical treatment are incomplete because of the limited number
of treated patients and that there is a risk of disease progression
during treatment or after initial response. Both oral and intrauterine
hormonal treatment are reported in literature [16].
Saegusa [17], suggested the use of progestins when positive
progesterone receptors are detected in well differentiated endometrial
cancer. Medroxyprogesterone acetate (400 mg/ day) and megestrol
acetate (160 mg / day) were the more frequently progestins used for
oral treatment.
Ricciardi [18] reported a study of 15 patients enrolled from May
2003 to December 2009 with early stage endometrial cancer or atypical
hyperplasia treated conservative therapy (medroxyprogesterone
acetate 500 mg/day - 1000 mg/day or megestrol acetate 80 mg/day -
160 mg/day) used for at least 12 weeks. The follow-up was performed
by hysteroscopic biopsy after one month starting the treatment, and
then repeated every three months until delivery. After delivery, the
follow-up was performed at 4, 8 and 12 months by hysteroscopic
biopsy. Of 15 women, 11 had complete remission and 4 of them
attained pregnancy with 4 live births. Three patients manifested
disease progression and received definitive surgery and one did not
have any response to treatment with further hysterectomy.
Mentrikoski [19] in a prospective study, assessed the outcome
of 30 women aged between 18 and 42 years, with complex atypical
hyperplasia and grade I, stage IA endometrial cancer conservatively
treated. Megestrol acetate (40 mg/day - 160 mg/day) was administered
and continued for 3 months. 23 women (77 %) had complete response,
persistence in 3 cases (10%) and progression in 4 cases (13%). The
mean to regression was 7.5 months in premenopausal women and
6.8 months in postmenopausal women. No significant difference was
noted in resolution status between pre- and postmenopausal women.
Yamazawa et al. [14] in a prospective study from 1999 to 2005,
assessed the outcome of nine women aged between 28 and 40 years,
with grade I, stage IA endometrial cancer conservatively treated. All
patients received progestins (medroxyprogesterone acetate 400 mg /
day) continued for 6 months. Seven women had complete response
and two of nine patients partially responded to treatment. Two
patients developed recurrent disease 10 and 22 months after the last
control (25% recurrence). In addition to this, of eight patients who
sought conceive, four had a pregnancy and three of them delivered.
The other aim of this study was to predict complete response
investigating expression pattern of five markers (IGF1R, PTEN,
progesterone receptor, estrogen receptor and ki67). This study
conclude that progesterone receptors are reliable markers to predict
complete response of endometrial cancer.
In a more recent experience, Chen [20] performed, between
January 2000 and December 2011, a retrospective study evaluating
the outcome of 53 patients with endometrial carcinoma, stage
IA, who underwent conservative treatment with progestin
(medroxyprogesterone acetate (MPA) at doses of 250 mg/day - 500
mg/day or Megestrol Acetate (MA) at doses of 160 mg/day – 480
mg/day. This study demonstrated the feasibility of fertility-sparing
strategies in women of childbearing age with Progesterone Receptors
(PR)- positive. Also, concluded that to decrease the risk of EC
recurrence, the identification of relevant factors that predict treatment
success is necessary. Obese patients (BMI ≥30) had a lower Complete
Remission (CR) rate to progestin treatment, and practitioners should
be particularly concerned about weight reduction in these patients. In
patients without a histologic response to progestin after 12 months,
the mode of therapy should be revaluated; these patients should be
counselled on the increased probability of failure.
Several studies are available in literature about progestinreleasing
IUD, as an alternative option to oral administration in the
conservative management of endometrial cancer. There are several
progestin-releasing IUDs, but the IUD that has been studied most
commonly for EC treatment is LNG-IUD that releases levonorgestrel
20 mcg/day (LNg20; Mirena). Progestin IUDs may be used for
treatment alone or in combination with an oral progestin.
In 2004, Montz [21] in a prospective study from 1999 to 2003
intrauterine progesterone appears to eradicate some cases of
presumed stage IA, grade 1 endometrioid cancer.
Cade [22] reviewed his experience of all patients receiving
intrauterine progesterone therapy for stage-1 endometrial cancer.
Of the 16 patients investigated, 10 patients (63%) responded to
treatment, with a median time to response of 5.5 months and the other
6 patients did not respond to treatment, but all were either early in
treatment or opted for surgical management before the average time
of response. Kim et al. [23], in 2012 demonstrated that intrauterine
progestin (Levonorgestrel) could be used in combination with oral
medroxyprogesterone in the conservative treatment of endometrial
cancer. Four of five treated patients and one of them had complete
and partial response respectively. All women had early stage disease
and follow-up lasted from 6 to 16 months.
A new fertility-sparing approach has been proposed in three
recent papers. Mazzon [24], included six patients with grade I,
stage IA endometrial cancer with positive progesterone receptor
underwent resectoscopic eradication of the lesion, ablation of
the closer endometrium and the underlying myometrium. After
hysteroscopy, all patients started oral progestins (megestrol acetate)
for 6 months. All patients had complete remission and four of them
achieved pregnancy.
Arendas [25] described two cases of stage IA endometrial
cancer managed conservatively by a combination of hysteroscopic
surgery and medical therapy for fertility-sparing purposes. One of
which achieved successful pregnancy using assisted reproductive
technology. They reviewed the existing literature on the use of
hysteroscopic resection in conservative management of endometrial
cancer to preserve fertility and concluded that hysteroscopic resection
to conservative management of early-stage endometrial carcinoma
may be a way to improve response and recurrence rates in women
wishing to preserve fertility.
Falcone [26] included 28 women, aged 18 to 40 years with early
stage of endometrial cancer. Hysteroscopic Resection (HR) was
performed to remove the tumour lesion, the endometrium adjacent
to the tumour, the myometrium underlying the tumour. If final
pathology confirmed a G1 endometrioid EC with no myometrial
invasion and PR positivity at immunohistochemistry, hormone
therapy was started 1 week after HR. Adjuvant hormonal therapy
consisted of oral progestins (megestrol acetate) or intrauterine
progestin device for 6 months. A complete response was observed in
96.3% of the patients 85.7% of patients achieved a durable complete
response, with a median duration of 95 months.
Follow up during conservative treatment
Although today no clear guidelines about follow-up for women
who undergo conservative treatment have been expressed, most
authors consider performing the first endometrial biopsy after three
months of hormonal therapy and then every three months until 24
months.
Chiva et al. [27] reported that a follow-up with endometrial
evaluation should be taken after 12 weeks of treatment. When a
positive biopsy occurs, another biopsy should be performed after 24
weeks of treatment. If the second biopsy results positive, a radical
treatment should be carried out. If the second biopsy results negative
the patient could start to attempt conception. Endometrial sampling
could be taken every 3 months.
Eskander et al. [28] suggested an endometrial evaluation
with curettage after three months of treatment. In case of disease
progression or persistence of cancer, he recommended hysterectomy.
If response to conservative management is confirmed, hormonal
therapy should be continued for 6 to 9 months.
Chen et al. [20] proposed a strict follow up with endometrial
evaluation 3 months after initiation of progestin treatment. Then it
recommended hysteroscopic and histologic evaluation every 3 months
until 12 months. In addition to this it considered the identification
of relevant factors that predict treatment success is necessary. Obese
patients had a lower CR rate to progestin treatment, Also, patients
without a histologic response to progestin after 12 months, the mode
of therapy should be revaluated; these patients should be counselled
on the increased probability of failure.
Falcone [26] recommended three months after starting
progestin therapy, patients entered the follow-up phase undergoing:
3-monthly general and gynecological examinations, transvaginal
ultrasonography (TVS), serum cancer antigen 125 (CA-125) and
diagnostic hysteroscopy with endometrial sampling.An abdomenpelvis
Computed Tomography (CT) is performed at 6 months and
6-monthly thereafter.
Definitive treatment after conservative treatment
Several authors suggested to performhysterectomy and bilateral
salpingo-oophorectomy with or without lymphadenectomywhen
patients have completed their fertility plans. A small risk of disease
progression after or during conservative treatment is described,
therefore hysterectomy should be considered after pregnancy. In
addition to this, for the high rate of synchronous ovarian cancer it
is recommended to complete the surgery with bilateral salpingooophorectomy
[16,27].
In 2012, Gallos [16] published a meta analysis of 32 studies of
women with endometrial cancer managed with fertility-sparing
treatment. He found a regression rate of 76.2% and a relapse rate of
40.6%. He concluded that because of the high rate of recurrence after
successful fertility-sparing therapy prophylactic hysterectomy with
bilateral salpingo-oophorectomy is the best option for patients who
have completed family planning [29,30].
Conclusion
Even though endometrial carcinoma is mainly diagnosed after
menopause, it may occur in young women as well and anytime a
young woman complains abnormal bleeding, endometrial carcinoma
should be ruled-out and all diagnostic tools should be used to exclude
the pathology. This is particularly important if risk factors for
endometrial carcinoma are present. This subject is important as the
number of younger women with endometrial cancer is rising because
of increasing obesity. The standard treatment for endometrial cancer
is total hysterectomy. In recent years, the surgical management
of the majority of endometrial cancers has become less extensive.
Hysterectomy removes the primary source of the cancer and allows
the assessment of the degree of local invasion. The ovaries are
removed to eliminate a source of estrogen, and to remove metastatic
or synchronous tumours. The role of lymph node dissection is
controversial. Although this procedure is usually performed in
women in menopause, conservative treatment could be a reasonable
option to propose to young patients with stage IA grade I endometrial
cancer and endometrial hyperplasia who desire to retain fertility.
An exhaustive evaluation of grade, stage, histology type, hormonal
receptors expression, myometrial invasion and metastatic diffusion
is necessary before introducing hormonal therapy as alternative
treatment. Today most of controlled studies about the conservative
treatment concerns patients with endometrial hyperplasia and
endometrioid adenocarcinoma grade I. Translation research in EC
cell lines has very recently yielded very interesting results regarding
the use of the antidiabetic drug metformin and its effect on EC cells.
These studies have shown that metformin suppresses EC cell growth
and exhibits an antiproliferative effect in women with EC and insulin
resistance. A prospective phase II study is announced and will further
elucidate the role of metformin in combination to progesterone and
active weigh management for the treatment of early stage EC in the
future.
There is often a debate when cancer is diagnosed in young
patients who wants to preserve fertility: risks and benefits of
conservative treatment should be widely discussed with patients. We
conclude, that nonsurgical approach is a valid option but indications
and eligibility of different therapies must be carefully considered and
strictly followed.
References
- Park JY, Nam JH. Progestins in the fertility-sparing treatment and retreatment of patients with primary and recurrent endometrial cancer. Oncologist. 2015; 20: 270–278.
- Rodolakis A, Biliatis I, Morice P, Reed N, Mangler M, Kesic V, et al. European Society of Gynecological Oncology Task Force for fertility preservation: clinical recommendations for fertility-sparing management in young endometrial cancer patients. Int J Gynecol Cancer. 2015; 25: 1258-1265.
- Park JY, Seong SJ, Kim TJ, Kim JW, Kim SM, Bae DS, et al. Pregnancy outcomes after fertility-sparing management in young women with early endometrial cancer. Obstet Gynecol. 2013; 121: 136-142.
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65: 87-108.
- Zivanovic O, Carter J, Kauff ND, Barakat RR. A review of the challenges faced in the conservative treatment of young women with endometrial carcinoma and risk of ovarian cancer. Gynecol Oncol. 2009; 115: 504-509.
- Crissman JD, Zoury RS, Barnes AE, Shellhas HF. Endometrial cancer in women 40 years of age or younger. Obstet Gynecol. 1981; 57: 699-704.
- Benshushan A. Endometrial adenocarcinoma in young patients: evaluation and fertility preserving treatment. Eur J Obstet Gynecol Reprod Biol. 2004; 117: 132-137.
- Duska LR, Garrett A, Rueda BR, Haas J, Chang Y, Fuller A. Endometrial cancer in women 40 years old or younger. Gynecol Oncol. 2001; 83: 388-393.
- Lee NK, Cheung MK, Shin JY, Husain A, Teng NN, Berek JS, et al. Prognostic factors for uterine cancer in reproductive-aged women. Obstet Gynecol. 2007; 109: 655– 662.
- Setiawan VW, Yang HP, Pike MC, McCann SE, Yu H, Xiang YB, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol. 2013; 31: 2607-2618.
- Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, Ledermann J, et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, Treatment and Follow-up. Int J Gynecol Cancer. 2016; 26: 2–30.
- Walsh C, Holshneider C, Hoang Y, Tieu K, Karlan B, Cass I. Coexisting ovarian malignancy in young women with endometrial cancer. Obstet Gynecol. 2005; 106: 693-699.
- Gitsch G, Hanzal EE, Jensen D, Hacker NF. Endometrial cancer in premenopausal women 45 years and younger. Obstet Gynecol. 1995; 85: 504-508.
- Yamazawa K, Hirai M, Fujito A, Nishi H, Terauchi F, Ishikura H, et al. Fertility-preserving treatment with progestin, and pathological criteria to predict responses, in young women with endometrial cancer. Hum Reprod. 2007; 22: 1953-1958.
- Navarria I, Usel M, Rapiti E, Neyroud-Caspar I, Pelte MF, Bouchardy C, et al. Young patients with endometrial cancer: how many could be eligible for fertility-sparing treatment? Gynecol Oncol. 2009; 114: 448-451.
- Gallos ID, Yap J, Rajkhowa M, Luesley DM, Coomarasamy A, Gupta JK. Regression, relapse, and live birth rates with fertility-sparing therapy for endometrial cancer and atypical complex endometrial hyperplasia: a systematic review and metaanalysis. Am J Obstet Gynecol. 2012; 207: e1-12
- Saegusa M, Hashimura M, Kuwata T, Okayasu I. Transcriptional regulation of pro-apoptotic Par-4 by NF-kappaB/p65 and its function in controlling cell kinetics during early events in endometrial tumourigenesis. J Pathol. 2010; 221: 26-36.
- Ricciardi E, Maniglio P, Frega A, Marci R, Caserta D, Moscarini M. Fertility-sparing treatment of endometrial cancer precursors among young women: a reproductive point of view. Eur Rev Med Pharmacol Sci. 2012; 16: 1934-1937.
- Mentrikoski MJ, Shah AA, Hanley KZ, Atkins KA. Assessing endometrial hyperplasia and carcinoma treated with progestin therapy. Am J Clin Pathol. 2012; 138: 524-534.
- Chen M, Jin Y, Li Y, Bi Y, Shan Y, Pan L. Oncologic and reproductive outcomes after fertility-sparing management with oral progestin for women with complex endometrial hyperplasia and endometrial cancer. Int J Gynaecol Obstet. 2016; 132: 34-38.
- Montz FJ, Bristow RE, Bovicelli A, Tomacruz R, Kurman RJ. Intrauterine progesterone treatment of early endometrial cancer. Am J Obstet Gynecol. 2002; 186: 651-657.
- Cade TJ, Quinn MA, Rome RM, Neesham D. Progestogen treatment options for early endometrial cancer. BJOG. 2010; 117: 879-884.
- Kim DH, Seong SJ, Kim MK, Bae HS, Kim M, Yun BS, et al. Dilatation and curettage is more accurate than endometrial aspiration biopsy in early-stage endometrial cancer patients treated with high dose oral progestin and levonorgestrel intrauterine system. J Gynecol Oncol. 2017; 28: e1.
- Mazzon I, Corrado G, Masciullo V, Morricone D, Ferrandina G, Scambia G. Conservative surgical management of stage IA endometrial carcinoma for fertility preservation. Fertil Steril. 2010; 93: 1286-1289.
- Arendas K, Aldossary M, Cipolla A, Leader A, Leyland NA. Hysteroscopic resection in the management of early-stage endometrial cancer: report of 2 cases and review of the literature. J Minim Invasive Gynecol. 2015; 22: 34-39.
- Falcone F, Laurelli G, Losito S, Di Napoli M, Granata V, Greggi S. Fertility preserving treatment with hysteroscopic resection followed by progestin therapy in young women with early endometrial cancer. J Gynecol Oncol. 2017; 28: e2.
- Chiva L, Lapuente F, González-Cortijo L, Carballo N, García JF, Rojo A, et al. Sparing fertility in young patients with endometrial cancer. Gynecol Oncol. 2008; 111: S101-S104.
- Eskander RN, Randall LM, Berman ML, Tewari KS, Disaia PJ, Bristow RE. Ferlility preserving options in patients with gynecologic malignancies. Am J Obstet Gynecol. 2011; 205: 103-110.
- Creasman WT, Mutch DE, Herzog TJ. ASTEC lymphadenectomy and radiation therapy studies: are conclusions valid? Gynecol Oncol. 2010; 116: 293-294.
- WHO. GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. 2012.