Case Report
Treatment of Recurrent Metastatic Breast Cancer in Pregnancy: A Case Report and Literature Review
Erin Shiv*, Jessica Shank, John Richard, Monica Lutgendorf, Salewa Oseni and Preston
Gable
Department of Obstetrics and Gynecology, Department of Obstetrics and Gynecology, Naval Medical Center San
Diego, USA
*Corresponding author: Erin N. Shiv, Department of Obstetrics and Gynecology, Naval Medical Center San Diego 34800 Bob Wilson Drive, San Diego, CA 92134, USA
Published: 24 Apr, 2017
Cite this article as: Shiv E, Shank J, Richard J, Lutgendorf
M, Oseni S, Gable P. Treatment of
Recurrent Metastatic Breast Cancer
in Pregnancy: A Case Report and
Literature Review. Ann Clin Case Rep.
2017; 2: 1432.
Abstract
Background: We present the case of a woman previously treated with surgery, adjuvant chemotherapy and radiation who was diagnosed with recurrent metastatic breast cancer during pregnancy.
Case: A 25-year-old woman was diagnosed with recurrent metastatic breast cancer during the
second trimester of pregnancy. Tamoxifen was initially tried, however she had progression of her
tumor markers, so was switched to a continuous infusion of 5-flurouracil (5-FU). She achieved a
partial response and delivered a viable infant without congenital malformations.
Conclusion: Many complex medical decisions occur when treating pregnant women with cancer.
Infusional 5-FU was used successfully in this patient with no effect noted in the fetus. This regimen
has not been previously described as treatment for recurrent breast cancer in pregnancy and should
be considered.
Keywords: Recurrent breast cancer; Pregnancy; Tamoxifen; 5-Flurouracil
Introduction
The incidence of cancer during pregnancy is approximately 1 in 1,000 [1]. The most common cancers in pregnancy are breast, cervical, leukemia, lymphoma, and melanoma, similar to those found in non-pregnant women of reproductive age [1]. The incidence of breast cancer during pregnancy is approximately 1 in 3,000 [1]. Little data exists regarding the optimal treatment of pregnancy-associated breast cancer, and even less so for recurrent breast cancer during pregnancy [2]. We present the case of a 25-year-old woman who was diagnosed with recurrent metastatic breast cancer during the second trimester of pregnancy. We will review her treatment course and discuss the literature regarding treatment options.
Case Presentation
A 25-year-old African-American Gravida 3 Para 1 was diagnosed with recurrent metastatic
breast cancer during the second trimester of pregnancy. In addition to her history of breast cancer,
her medical history was significant for obesity (body mass index of 30), glucose-6-phosphatedehydrogenase
deficiency, and angioedema of unknown etiology. She was initially referred to
hematology/oncology for evaluation of angioedema with suspicion for systemic mastocytosis, after
having two episodes of hives and facial swelling that required intubation and intensive care unit
admission for several days. A bone marrow biopsy at that time was suboptimal, but showed a small
population of mast cells (3%) by flow cytometry. During the evaluation of angioedema, the patient
noted a grape-sized mass in the upper outer quadrant of the left breast. A breast ultrasound revealed
a 1 centimeter (cm) mass; a core biopsy revealed invasive ductal carcinoma. She underwent a left
breast mastectomy with sentinel node biopsy, which showed a 4 cm tumor with 3 of 3 sentinel nodes
positive for carcinoma, and was ultimately diagnosed with Stage IIb invasive ductal carcinoma of
the breast (T2N1aMx, grade 2, estrogen and progesterone receptor positive [ER/PR+], HER-2
negative). Further staging with a positron emission tomography and computed tomography (PET/
CT) did not reveal any distant disease. She desired future fertility and underwent oocyte retrieval
before starting adjuvant chemotherapy with doxorubicin 60 mg/m2 and cyclophosphamide 600
mg/m2 intravenously (IV) every 2 weeks for 4 cycles followed by paclitaxel 175 mg/m2 IV every 2
weeks for 4 cycles. After her course of adjuvant chemotherapy, a completion axillary dissection was
performed. No residual disease was seen and this was followed by chest wall and axillary radiation (50 gray in 25 fractions to the chest wall and axilla with an additional
10 gray in 5 fractions as a scar boost). Shortly after finishing radiation
therapy, she became pregnant and subsequently miscarried in the first
trimester. At that time, the recommendation was made to postpone
future pregnancies and start endocrine therapy with tamoxifen (20mg
orally daily) and goserelin (3.6mg subcutaneously every 28 days).
However, she strongly desired pregnancy and declined endocrine
therapy.
Several months later, she became pregnant again. At 14 weeks
gestation she presented to the emergency department with acute onset
of severe low back pain. She was admitted to the hospital for pain
control for 3 days. An X ray of the lumbar spine showed no osseous
lesions. Nine days later she was readmitted for uncontrolled pain.
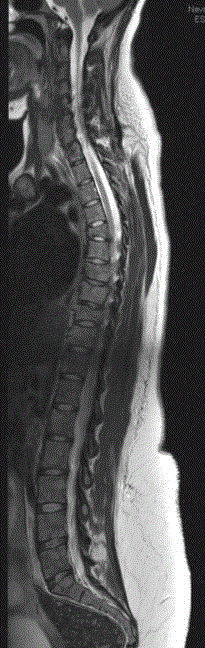
A magnetic resonance image (MRI) of the spine showed multiple
foci of heterogeneous bright T2 signal and dark T1 signal within the
vertebral bodies, suspicious for metastatic breast cancer (Figure 1). A
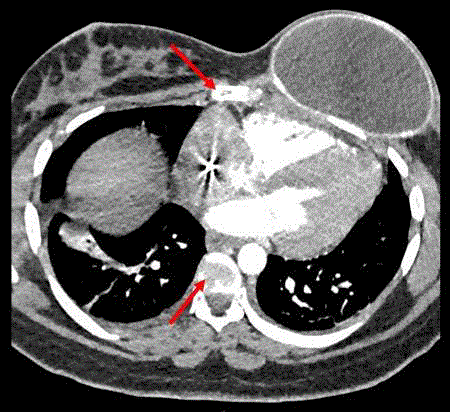
chest CT scan performed around the same time also showed diffuse
lytic lesions throughout her spine (Figure 2). This was confirmed with
an image guided biopsy of the L3 vertebra. A multidisciplinary team consisting of oncology, neonatology, and maternal-fetal medicine
physicians reviewed the case and recommended treatment with
tamoxifen. Tamoxifen was started at 16 weeks gestation. Serial CA
15-3 measurements were obtained and increased from 83.5 to 126.9
over the course of 4 weeks. Additionally, she developed worsening
pain, anemia and thrombocytopenia within the first month of
treatment with tamoxifen. A follow-up MRI of the spine showed
progressive metastatic disease involving nearly every bone, which
was thought to account for her significant cytopenias. A second
multidisciplinary meeting was held and the recommendation was
for her to switch treatment to a continuous infusion of 5-fluorouracil
(5-FU) (250 mg/m2/day by continuous IV infusion for 21 days,
followed by 7 days of no treatment). This was started at 21 weeks
gestation and was continued until delivery for a total of 3 cycles. For
the remainder of her pregnancy, despite incrementally increasing
high dose narcotics, she was repeatedly admitted for control of pain,
nausea, and constipation. Her CA 15-3 improved from 126.9 to 41.3
while on 5-FU.
Due to concerns for preterm labor, she received a course of
betamethasone (12.5 mg intramuscular, 2 doses 24 hours apart) for
fetal lung maturation at 27 weeks. A rescue course was completed 24
hours prior to her planned delivery at 34 weeks gestation. She was
not a candidate for neuraxial anesthesia due to her spinal metastases,
so the recommendation was made for delivery via primary cesarean
with bilateral salpingo-oophorectomy (due to ER/PR+ tumor) under
general anesthesia or induction of labor with Remifentanil patientcontrolled
analgesia. The patient elected for induction of labor and
underwent an uncomplicated spontaneous vaginal delivery with
post-placental placement of a Paraguard intrauterine device for
contraception. A 1740 gram male infant was born without obvious
congenital malformations. Due to the high doses of narcotic
medications necessary to control pain during pregnancy, the infant
was treated for neonatal abstinence syndrome in the neonatal
intensive care unit and was discharged home in good condition on
day of life 45. The patient’s postpartum course was significant for
hospital readmission on postpartum day 6 for endomyometritis
that was treated with antibiotics until resolution of fever and fundal
tenderness.
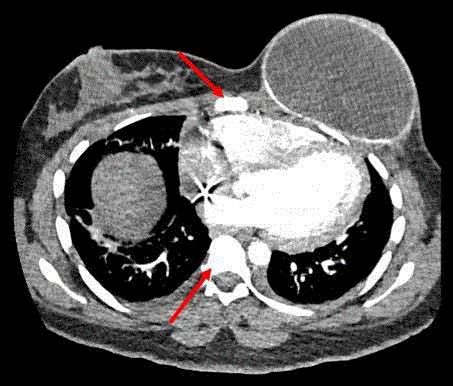
After delivery, PET/CT scan showed no evidence of solid
organ metastases, with diffuse bony sclerosis, consistent with
a partial response to 5-FU (Figure 3). At 3 weeks postpartum,
she was started on capecitabine (2000 mg orally, every 3 weeks)
and denosumab (120 mg subcutaneously monthly). Denosumab
was stopped due to hypocalcemia. At 17 weeks postpartum she underwent an uncomplicated laparoscopic bilateral salpingooophorectomy.
Pathology showed malignancy on pelvic washings
with micrometastases on the bilateral ovaries. Follow-up CT at that
time showed diffuse bony sclerosis, without obvious other areas
of involvement. After surgery capecitabine was stopped due to
progressive increase in CA15-3 levels and fulvestrant was initiated.
Figure 1
Figure 2
Figure 3
Figure 3
Chest CT at 3wks postpartum showing interval resolution of lytic
lesions in vertebral bodies.
Discussion
Breast cancer is most commonly treated with a multi-modality
approach including chemotherapy, radiation, and surgery. However,
radiation is contraindicated in pregnancy and surgery is not useful in
patients with recurrent multi-focal metastatic disease.
The initial treatment for our patient was tamoxifen, a non-steroidal,
anti-estrogen medication that is used to treat ER+ breast cancers.
The effects of tamoxifen on pregnancy are not well understood, and
most information comes from animal studies and case reports. The
majority of information regarding fetal effects of tamoxifen exposure
comes from case reports and the AstraZeneca Safety Database. A
review article of this database by Braems et al. [3], describes 15 cases of
fetal tamoxifen exposure after the first trimester, 3 resulting in fetuses
with congenital anomalies. In general, the recommendation is to stop
tamoxifen prior to pregnancy and delay conception for 2 months due
to the long half-life of the drug [3]. In this case, the fetus was exposed
to tamoxifen from 16 weeks to 22 weeks gestation and to date, no
congenital anomalies have been noted. Unfortunately, our patient
did not respond to this treatment, likely due to the very high levels
of estrogen during pregnancy. Estradiol levels of a non-pregnant
premenopausal woman can reach up to 400 pg/mL, depending on
the time in her menstrual cycle. During pregnancy, estradiol levels
can be greater than 7,000 pg/mL [4]. There is little data discussing
the efficacy of tamoxifen during pregnancy, given the high estradiol
levels. Our experience would suggest tamoxifen is of little benefit in
this setting.
Traditionally, chemotherapy is avoided in the first trimester due
to the 14% risk of major malformations versus the baseline risk of 3%
in the general population in the United States [5]. Treatment with
cytotoxic chemotherapy in the second or third trimester appears to
be safer with a risk of major fetal malformation similar to baseline,
although the rate of stillbirth is higher (2% versus 0.3%) [5]. Choosing
treatment for a pregnant patient with recurrent metastatic breast
cancer who has been previously treated is challenging. In the case
of this patient, the challenge was selecting a chemotherapy regimen
that did not include agents that she had previously been exposed to
(doxorubicin, cyclophosphamide, and paclitaxel). Berry published
a standardized protocol for breast cancer during pregnancy that
utilizes a multidisciplinary team approach, including surgery for
those women with operable disease and systemic chemotherapy with
cyclophosphamide, doxorubicin, and 5-fluorouracil (FAC regimen).
In their study of 22 women, no unusual neonatal complications were
seen. [2]. Our patient had previously been treated in the adjuvant
setting with doxorubicin and cyclophosphamide, but not with
fluorouracil. According to Lambertini et al, fetal exposure to 5-FU
in the first trimester can cause spontaneous abortion and major
malformations including skeletal defects. However, second trimester exposure has not been associated with birth defects [5]. In a nonpregnant patient, a reasonable alternative to 5-FU may have been
capecitabine, an oral 5-FU prodrug, although this medication has
been linked to teratogenicity in animal studies. Continuous infusion
of 5-FU as treatment for advanced breast cancer was first described
by Huan et al in 1989 [6]. Later, Regazzoni et al. [7]. described it as
a well-tolerated and effective treatment for metastatic breast cancer
that could be infused in the outpatient setting. 5-FU was chosen
in our patient because of its relative safety in pregnancy and it was
not a medication to which she had been previously exposed. To
our knowledge, this is the first reported case where the continuous
infusion of 5-FU was used to treat recurrent metastatic breast cancer
in a pregnant patient. Our patient responded well to 5-FU as noted
by the decrease in the CA 15-3 level from 126.9 to 41.3 as well as
improvements in pain, anemia and thrombocytopenia. Additionally,
no effects have been noted in the infant thus far.
Many complex medical decisions occur when treating pregnant
women with cancer including treatment choice, symptom control
and timing and route of delivery. 5-FU was used with success in
this patient. This medication has not been previously described as a
treatment option for recurrent breast cancer in pregnancy and should
be considered.
Teaching Points
1. Cancer recurrence and metastasis should be part of the
differential for all pregnant patients presenting with pain and a
history of cancer.
2. Continuous infusion of 5-fluorouracil should be considered
for pregnant patients with recurrent breast cancer.
3. Tamoxifen is generally not effective during pregnancy due
to high estrogen levels.
References
- Pavlidis NA. Coexistence of pregnancy and malignancy. Oncologist. 2002; 7: 279-287.
- Berry DL, Theriault RL, Holmes FA, Parisi VM, Booser DJ, Singletary SE, et al. Management of breast cancer during pregnancy using a standardized protocol. J Clin Oncol. 1999; 17: 855-61.
- Braems G, Denys H, De Wever O, Cocquyt V, Van den Broecke R. Use of tamoxifen before and during pregnancy. Oncologist. 2011; 16: 1547-1551.
- Abbassi-Ghanavati M, Greer LG, Cunningham FG. Pregnancy and laboratory studies: a reference table for clinicians. Obstet Gynecol. 2009; 114: 1326-1331.
- Lambertini M, Kamal NS, Peccatori FA, Del Mastro L, Azim HA Jr. Exploring the safety of chemotherapy for treating breast cancer during pregnancy. Expert Opin Drug Saf. 2015; 14: 1395-408.
- Huan S, Pazdur R, Singhakowinta A, Samal B, Vaitkevicius VK. Low-dose continuous infusion 5-fluorouracil. Evaluation in advanced breast carcinoma. Cancer. 1989; 63: 419-22.
- Regazzoni S, Pesce G, Marini G, Cavalli F, Goldhirsch A. Low-dose continuous intravenous infusion of 5-fluorouracil for metastatic breast cancer. Ann Oncol. 1996; 7: 807-813.