Case Report
A Case of Toxic Hepatitis and Acute Liver Failure Induced by Ingestion of Raw Sansevieria
Joo Hyun Sohn1,3*, Ji Yeoun Kim1, Tae Yeob Kim1, Jae Yoon Jeong1, Sun Min Kim1 and Ju Yeon Pyo2
1Department of Internal Medicine, Hanyang University Guri Hospital, Hanyang University, Korea
2Department of Pathology, Hanyang University Guri Hospital, Hanyang University, Korea
3Department of Gastroenterology, Hanyang University College of Medicine, Korea
*Corresponding author: Joo Hyun Sohn, Department of Internal Medicine, Hanyang University Guri Hospital, 153 Gyeongchun-ro, Guri 471- 701, Korea
Published: 03 Sep, 2017
Cite this article as: Sohn JH, Kim JY, Kim TY, Jeong
JY, Kim SM, Pyo JY. A Case of
Toxic Hepatitis and Acute Liver
Failure Induced by Ingestion of Raw
Sansevieria. Ann Clin Case Rep. 2017;
2: 1429.
Abstract
Herbal medicines are frequently used in the oriental region to treat a variety of disease and symptoms. Nearly 50% of patients with toxic hepatitis are related to these herbs and health supplements in Korea. We report, for the first time, a case of liver failure due to toxic hepatitis induced by Sansevieria ingestion. The patient took raw Sansevieria for a month and thereafter developed symptoms such as jaundice, nausea, anorexia and general weakness. The diagnosis was made after a thorough history taking, laboratory exams, imaging studies such as ultrasonography, liver biopsy, and the exclusion of other causes of hepatitis. We administered supportive treatment for acute toxichepatitis but the patient’s symptoms and liver functions worsened and finally expired in 2 months. Since toxic hepatitis is frequently induced by taking herbs and health supplements and occasionally fatal, it is crucial to make early diagnosis and immediately stop each drug which is suspected to cause liver injury. This case is noteworthy because Sansevieria assumed to have hepatoprotective effect but is the first case reported for its fatal hepatotoxicity.
Keywords: Liver failure Acute; Drug-induced liver injury; Sansevieria; Toxic hepatitis
Introduction
Incidence of drug-induced liver disease (DILD) is from 2.3 up to 19.1 per 100,000 inhabitants per year, especially higher with older age and it is one of the leading causes of acute liver failure [1]. As concerns for health are growing, the use of herbs and health supplements are increasing worldwide
and especially in Korea as an easier way to care for health [2]. In Korea, over 50% of patients with acute hepatitis are caused by drugs or toxins and 42-74.5%of the causes of toxic hepatitis were
occupied by oriental medicines and other herbs [3-5]. Furthermore, patients with acute liver failure induced by idiosyncratic drug reactions have poor prognosis, with 60% to 80% mortality without
a liver transplantation [3,4,6]. In most cases, there are no effective drugs or antidotes other than stopping the causative agent and providing supportive care [7,8].
Sansevieria is assumed to have some benefits on the liver and we could not find any reports
about hepatictoxicity of raw Sansevieria [9]. For the first time, we report a case of toxic hepatitis caused by consuming Sansevieria for a month in a 79 years old female, which ultimately led to
progressive liver failure and death in 2 months.
Case Presentation
In August 2012, a 79-year-old female with no other underlying chronic liver disease visited
emergency room with newly developed jaundice and was admitted. About six months prior to her
visit, she underwent percutaneous transhepatic biliary drainage and percutaneous transhepatic
gallbladder drainage with balloon dilatation for calculous cholecytitis and cholangitis. After
the procedure and treatment of antibiotics, her cholecystitis and cholangitis were improved and
then the patient and her family refused operation. Thereafter ursodeoxycholic acid (UDCA) was
prescribed for several months with no recurrence of clinical symptom and signs of cholecystitis or
cholangitis. Several weeks before this admission, she arbitrarily stopped taking UDCA and instead
she insisted on taking raw Sansevieria for a month, which was believed, without proven efficacy, to
have hepatoprotective effect. She denied taking other medicines or herbs and alcohol drinking. She
also denied any abrupt or colic abdominal pain and febrile illness during the past several months.
On physical examination, she was icteric and ill looking. Initial
vital signs at emergency room were: blood pressure of 120/70 mmHg,
pulse of 72 beats per minute, respiratory rate of 20 breaths per minute,
and temperature of 36.8oC. Mild right upper quadrant discomfort
with tenderness was noted.
The laboratory tests were as follows: hemoglobin 11.8 g/dL, WBC
3,400/mm3 (segment 72%, lymphocytes 22%, and eosinophil 1%),
platelet 140,000/mm3, serum AST/ALT ratio 9.9 (797/79 U/L), r-GTP
295 U/L, ALP 140 U/L, bilirubin (total/direct) 14.2/8.8 mg/dL, LDH/
CPK 726/39 (U/L). Prothrombin time INR was 1.38. Serologic tests
for viruses causing acute hepatitis such as HAV Ab IgM, HBs Ag, anti-HBc IgM, HCV Ab IgG, HCV RNA PCR, HEV Ab IgM, HEV Ab
IgG, CMV Ab IgM, CMV Ab IgG, HSV Ab IgM, HSV Ab IgG, EBV
Ab IgM, and EBV Ab IgG were all negative. Immunological profiles
for anti-smooth muscle antibody, anti-nuclear antibody, and antimitochondria
antibody were also all negative. There were no bacterial
growth in blood and urine.
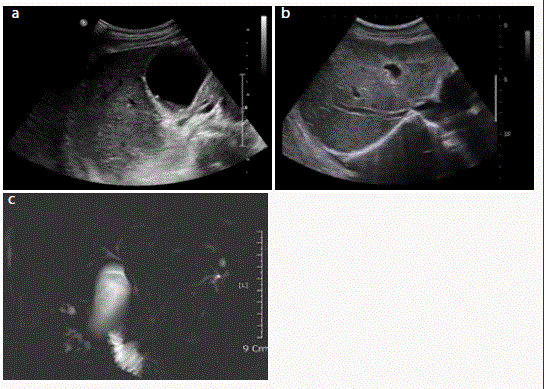
Ultrasonography examination showed bile sludge and small
gallstones in gallbladder without gallbladder wall thickening,
heterogeneously increased hepatic parenchymal echogenicity and no
signs of chronic liver disease (Figure 1A and B). In abdominal contrast
enhanced computed tomography showed no stones in common bile
duct but pneumobilia due to previous balloon dilatation and did not
show any enlarged lymph nodes or hepatosplenomegaly. Magnetic
resonance cholangiogram also showed stones in the gallbladder, but
did not give any evidence for cholecystitis and any obstructive lesion
such as bile sludge and stones in the common bile duct (Figure 1C).
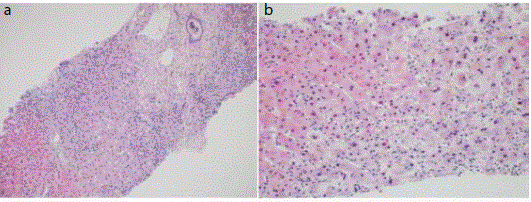
On her 17th day after admission, transjugular liver biopsy
was performed to exclude other causes of hepatitis. Histological
examination showed severe hepatocyte degeneration with piecemeal
necrosis and severe cholestasis. There were severe lobular and portal
inflammation but no portal fibrosis was found (Figure 2). This result
is compatible with severe acute hepatitis with no underlying chronic
liver disease.
Based on RUCAM score of 6 with cholestatic pattern, [10] she
was diagnosed with toxic hepatitis and supportive treatment such as
UDCA and steroid for cholestasis were given in parallel with sufficient
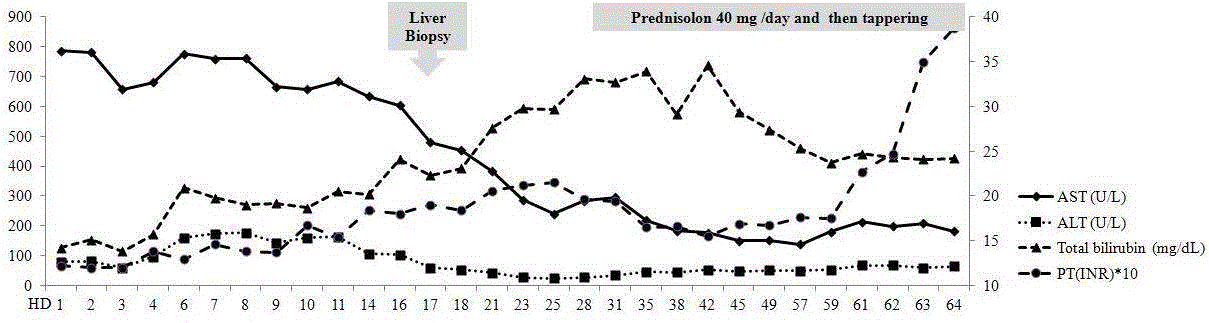
calories and nutritional supply. Despite of 2 months with supportive
treatment, her laboratory examinations did not show improvement
(Figure 3). Her anorexia, general weakness and jaundice worsened and
later she developed mental change caused by hepatic encephalopathy.
Finally, she expired due to progression of liver failure.
Figure 1
Figure 1
Imaging study of the patient. (A) Ultrasonographic image
showsbile sluge and small gallstones in gallbladder without gallbladder wall
thickening. (B)It also shows heterogeneously increased hepatic parenchymal
echogenicity and no signs of chronic liver disease. (C) Magnetic resonance
cholangiogram shows no obstructive lesion or duct dilatation in common bile
duct.
Figure 2
Figure 2
Microscopic findings of liver biopsy specimen. (A) Histologic
examination shows extensive piecemeal necrosis and severe lobular and
portal inflammation but no portal fibrosis (H&E, x100). (B) There is severe
hepatocyte ballooning degeneration with apoptosis and cholestasis (H&E,
x200).
Figure 3
Figure 3
Clinical course of the patient.
HD: Hospital Day; PT: Prothrombin Time; INR: International Normalized Ratio
Discussion
DILD is an increasing health problem, especially in the oriental
region and is one of the leading causes of acute liver failure in
paralleling with growing concerns for health and increasing use of
herbs and health supplements [2]. Recently it was reported that over
50% of patients with acute hepatitis are caused by drugs and 42-74.5%
were caused by oriental medicines and other herbs in Korea [3-5].
As we know, this case is the first to report the hepatotoxicity
of raw Sansevieria, finally leading to death. There are few reports
showing improvement of elevated liver enzyme in rats by Sansevieria
[9]. However, clinical effectiveness is not sufficiently defined with
large and randomized studies. Also we could not find any reports about hepatic toxicity of raw Sansevieria.
Diagnosis of DILD is very difficult because of the absence of
definitive diagnostic tests and rare incidence. Histopathology is not
useful to find the etiology but only shows the type and degree of
hepatic injury. Therefore, the key to diagnose is to assess the temporal
relationship between drug initiation and development of an abnormal
liver test and to exclude other causes of liver diseases [8]. That is why
detailed history taking and physical exams, laboratory findings and
histopathology are needed to diagnose. In this case, we excluded
other common causes of liver disease such as various hepatitis
viruses, rare non-hepatitis viruses, and autoimmune liver diseases.
She was hemodynamically stable before and during the admission,
so ischemic reperfusion or anoxic liver injury could be excluded.
She had known gallbladder stones but she denied any abdominal
pain and there were no signs of cholecystitis and cholangitis by
ultrasonography, computed tomography and magnetic resonance
cholangiography. So the possibility of biliary stone passage was low
and furthermore it could be excluded by her liver histopathology and
clinical course.
As aforementioned, biochemical index for diagnosing toxic
hepatitis is not established and it is not ethically allowed to artificially
induce or put injured liver cell into the human body. So evaluation
is usually based on the causality assessment method, especially the
RUCAM scale [10]. In this case, the patient showed cholestatic-type
hepatic injury with R value <2. Based on the RUCAM score, our
case was classified ‘probable’ for a total of 6 points in combination
of following factors; time to (+2), course (0), risk factors (+2),
concomitant drugs (0), exclusion of other case of liver injury (+2),
previous information on hepatotoxicity (0), and response to re
administration (0).
In treating DILD, most important therapy is to withdraw the
suspected agent. There are no other beneficial therapies reported
except for the use of N-acetylcysteine in acetaminophen toxicity
[11]. Corticosteroid may be used in DILD cases with evident
hypersensitivity, but there are no proven benefits [12]. Using UDCA
for cholestatic liver injury is also controversial [8]. In this case we
used every method known but the results were fatal.
It is notable that according to a recent article, her fatal destiny
might be assumed by her initial lab results showing high AST/ALT
ratio (9.9) and hyperbilirubinemia (14.2 mg/dL).In that report, it was
identified AST but not ALT to be an independent predictor of bad
outcome and it was also pointed out that the AST and bilirubin levels
are the most important predictors of death or liver transplantation in
severe DILD [13]. It is also in parallel with another result in patients
with DILD showing higher aminotransferase levels, especially AST
in patients who progressed to fulminant hepatic failure compared
with those who did not [14]. Furthermore, it is also in line with an
observation that showed a higher AST/ALT ratio in fatal cases than in
survivors with severe acute viral hepatitis [15]. In addition, we could
fully exclude other causes of high ratio of AST/ALT such as alcoholic
hepatitis, Wilson disease, cirrhosis, acute fatty liver of pregnancy,
rhabdomyolysis, etc.
With increasing interests in health and usage of herbal medicine,
also the risks of developing DILI also is increasing. But there are few
data regarding the incidence and clinical manifestation about DILI induced by herbal medicine. We are reporting for the first time
with a case of Sansevieria induced toxic hepatitis, which was alleged
to be hepatoprotective, to arouse that toxic hepatitis can occur by
Sansevieria. Although most of the patients do not regard herbal
medicines or dietary supplements as medicine and do regard those
as not toxic material, physicians must keep in mind that any health
supplements such as Sansevieria can cause hepatotoxicity, especially
with high suspicion.
In conclusion, we report a case of toxic hepatitis caused by
consuming Sansevieria for a month in a 79 years old female which
ultimately led to progressive liver failure and death in 2 months.
References
- Bjornsson ES, Bergmann OM, Bjornsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013; 144: 1419-1425.
- Park HA. Top 10 dietary supplements of korean adults from the 4th Korea national health and nutrition examination survey. Korean J Fam Med. 2011; 32: 263-266.
- Kang SH, Kim JI, Jeong KH, Ko KH, Ko PG, Hwang SW, et al. [Clinical characteristics of 159 cases of acute toxic hepatitis]. Korean J Hepatol. 2008; 14: 483-492.
- Kim JB, Sohn JH, Lee HL, Kim JP, Han DS, Hahm JS, et al. Clinical characteristics of acute toxic liver injury. Korean J Hepatol. 2004; 10: 125-134.
- Suk KT, Kim DJ, Kim CH, Park SH, Yoon JH, Kim YS, et al. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol. 2012; 107: 1380-1387.
- Hoofnagle JH, Carithers RL Jr, Shapiro C, Ascher N. Fulminant hepatic failure: summary of a workshop. Hepatology. 1995; 21: 240-252.
- Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med. 2006; 354: 731-739.
- Verma S, Kaplowitz N. Diagnosis, management and prevention of drug-induced liver injury. Gut. 2009; 58: 1555-1564.
- Chigozie IJ, Chidinma IC. Positive moderation of the hematology, plasma biochemistry and ocular indices of oxidative stress in alloxan-induced diabetic rats, by an aqueous extract of the leaves of Sansevieria liberica Gerome and Labroy. Asian Pac J Trop Med. 2013; 6: 27-36.
- Danan G, Benichou C. Causality assessment of adverse reactions to drugs--I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993; 46: 1323-1330.
- Bunchorntavakul C, Reddy KR. Acetaminophen-related hepatotoxicity. Clin Liver Dis. 2013; 17: 587-607.
- Suk KT, Kim DJ. Drug-induced liver injury: present and future. Clin Mol Hepatol 2012; 18: 249-257.
- Bjornsson E, Olsson R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology. 2005; 42: 481-489.
- Ohmori S, Shiraki K, Inoue H, Okano H, Yamanaka T, Deguchi M, et al. Clinical characteristics and prognostic indicators of drug-induced fulminant hepatic failure. Hepatogastroenterology. 2003; 50: 1531-1534.
- Gitlin N. The serum glutamic oxaloacetic transaminase/serum glutamic pyruvic transaminase ratio as a prognostic index in severe acute viral hepatitis. Am J Gastroenterol. 1982; 77: 2-4.