Case Report
A Mediterranean Spotted Fever Case in a Febrile Trombocytopenia Patient with the Presumptive Diagnosis of Crimean-Congo Haemorragic Fever
Burcu Uysal1, Tulin Demir2,3*, Bekir Celebi3, Bulent Acar3 and Selcuk Kılıc3
1Department of Infectious Disease, Ahi Evran University, Turkey
2Department of Clinical Microbiology, Ahi Evran University, Turkey
3Public Health Institution, Microbiology Reference Laboratories, Turkey
*Corresponding author: Tulin Demir, Public Health Institution of Turkey, Microbiology Reference Laboratories, Ankara, Turkey
Published: 21 Aug, 2017
Cite this article as: Uysal B, Demir T, Celebi B, Acar B,
Kılıc S. A Mediterranean Spotted Fever
Case in a Febrile Trombocytopenia
Patient with the Presumptive Diagnosis
of Crimean-Congo Haemorragic Fever.
Ann Clin Case Rep. 2017; 2: 1421.
Abstract
Background: Mediterranean Spotted Fever (MSF) is a tick-borne zoonotic infection caused by Rickettsia conorii. It is commonly transmitted to humans by dog ticks, Rhipicephalus sanguineus.
The infection mainly occurs from spring to summer and characterized with fever, headache, myalgia,
maculopapuler rash and an inoculation escar at the site of the tick bite. The diagnosis of the disease
is difficult due to the asymptomatic clinical features and symptoms, and can lead to misdiagnosis
with many disease presented with fever and rash. Laboratory findings are also nonspecific but
trombocytopenia, increase in the levels of transaminases, hyponatremia was observed in majority
of the cases.
Case Report: In this report we report a ABA case in a 35-year-old male patient, dealt with animal
husbandry in an urban province presented with arthralgia, fever, malaise, headache. Patient serum
was tested for Crimean-Congo Haemorrhagic Fever (CCHF) by PCR and IFA IgM/G as the clinical
features and medical history was compatible with CCHF and negative results were obtained. While
patient sera was negative for Coxiella burnetti by IFA, IgM 1/96, IgG 1/160 titers was detected for
R.conorii. The patient received a ten day course of doxycycline, with complete resolution of all
symptoms without any complication.
Conclusion: Viral diseases presented with fever and rash, allergic reactions, drug erupsions, typhoid
fever, leptospirosis, erlichosis, anaplasmosis and CCHF should be considered in the differential
diagnosis and MSF diagnostic testing has to be included in febrile illness with thrombocytopenia,
even in the absence of an eschar or a tick bite or rash.
Keywords: Rickettsiosis; Trombocytopenia; Tache noir; Immunflorescent antibody test
Introduction
Rickettsia is a genus of gram-negative, nonmotile and nonspor-forming bacteria classified into
four groups including “spotted fever”, “typhus”, “Rickettsia bellii group” and “Rickettsia canadensis”
based on serology [1,2]. Mediterranean Spotted Fever (MSF) also known as “boutonneuse fever”, related with Rickettsia conorii. The major vector and potential reservoir is Rhipicephalus sanguineus,
the brown dog tick [1]. Additionally, Rh. evertsi, Rh. simus, Rh. mushamae, Ixodid ticks and Rh. bursa is reported as vectors [1,3]. The incidence of the disease has a seasonal variation related with tick activity mostly occuring between May to September. Also, incidence can be higher due to high temperatures, decrease in rainfall and number of frosty days in the previous year [1].
The disease is characterized with fever, headache, myalgia, maculopapuler rash and typical
inoculation escar at the tick-bite site followed by an incubation period of 2-14 days [1]. Symptoms are nonspesific and confused with many diseases characterized with fever and rash, making the
diagnosis more difficult. Laboratory findings are also nonspecific but leucopenia, trombocytopenia,
increase in transminases, decrease in the serum levels of Na, K, Cl may be seen in most cases [1,4].
The disease is endemic in Mediterranean region including South Europe and North Africa.
Cases were also reported from Bulgaria, Ukraine, North and Central Europe, and India [1,2]. In Turkey, the first cases were reported from Thrace region [5]. Although some cases were confirmed by serological tests for Ricketsiosis, many of them were underdiagnosed due to lack of using diagnostic tests especially in patients outside the area of endemicity or during
winter [6]. In this report, we evaluate a MSF case with the presumptive
diagnosis of CCHF presented with febrile trombocytopenia but no
rash.
Case Presentation
A 35-year old male with fever, headache, generalized myalgia
for four days was admitted to a tertier hospital in May 2015. The
patient was working in an animal husbandry and living in a rural
area located in the central Anatolia. He was febrile (38.5°C) with a
pulse rate of 96/min, and blood pressure 140/80 mm/Hg. Rash was
not detected and other system examinations were normal. The patient
revealed a tick bite prior from the symptoms and removed the tick
with his hands and might have been exposed to the excretions of the
animals. A black-crusted lesion in a diameter of 2-3 cm surrounded
by hyperemic area was observed in the tick-bite area in the sculp
(Figure 1). Possible infection sources were evaluated. Additionally,
serum sample of patient were send to the Public Health Institution
of Turkey, Microbiology Reference Laboratory with the presumptive
diagnosis of CCHF. Laboratory tests revealed minimal decrease in
hemoglobin (11.9 gr/dl), hematocrite (39.4%), trombocyte (60000/
mm3) and minimal increase in CRP (11.8 mg/dL), sedimentation
(25 mm/h). Electrolite levels were normal range except decrease
in sodium level (131 mEq/L). Blood samples were also tested for
serological markers of viral hepatitis, cytomegalovirus, Epstein-Barr
virus, brucellosis, salmonellosis, toxoplazmosis and were all negative.
Patient sera was tested for CCHF by RT-PCR (Altona Diagnostics,
Germany) and IFA IgM/IgG (Euroimmun, Germany) and were
negative. As the patient history revealed rural residence, dog feeding,
removal of the dog ticks with his hands and tick bite history and the
clinical signs, symptoms of the patient and lesion in the sculp sera was
tested with R. conorii IFA (Focus Technologies, USA) and C. burnetii
Phase I-II (Vircell SL, Spain) IFA IgM/IgG. Sample was negative for
C. burnetii but R. conorii IFA were positive for IgM 1/96 and IgG
1/160 titer. Convalescence sera taken after two weeks from the acute
sample, R. Conorii IFA IgM was 1/384 and IgG 1/640. Detection of R.
conorii by PCR from escar was not performed as the patient consent
was not received.
Evaluation of the case by Raoult et al. [4] MSF diagnostic
criteria; revealed a score of 45 with the following parameters of;
occurence in spring, exposure to dog ticks, presence of fever, escar,
trombocytopenia and four fold increase in the R. conorii specific
antibody titers between two serum samples (Table 1). The patient was given doxycycline (2 x 100 mg) empirically. At the third day of the
theraphy, fever was decreased, headache, myalgia was resolved and
increase in trombocytes (142000/mm3) were observed and the patient
was recovered with complete cure. Additional informed consent
was obtained from all individual participants for whom identifying
information is included in this article.
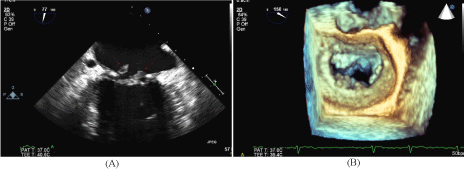
Figure 1
Figure 1
Trans-esophageal echocardiogram following stroke.
(A) 2-chamber view of the mitral valve in systole with vegetations attached to
the mitral annuloplasty ring.
(B) 3D TEE image of the mitral valve in diastole with multiple small vegetations
attached to the mitral annuloplasty ring.
Discussion
R. conorii grows in the salivary gland of the ticks, particularly, Rh.
sanguineus and other Rhipicephalus species including Rh. bursa, Rh.
evertsi, Rh. simus, Rh. mushamae and Ixodid species. The bacteria is
transmitted to the human during the feeding of the ticks, and as a result
of contact with the rodent carcasses while cleaning the weeds [6,7].
Nonspecific clinical, laboratory symptoms along with the lack of escar
and rash makes the diagnosis difficult. Although tache noir and rash is
characteristics, it may not be observed in all cases [1]. Hyponatremia
can be an important diagnostic clue for the physician as it can be the
only abnormallity in most cases [8-10]. Two methods are used in the
laboratory diagnosis of MSF including direct identification (isolation
of the bacteria from samples or identifing bacteria with molecular
techniques) and indirect diagnosis (specific IgM and IgG detection)
[2]. IFA is considered as reference method with the IgM titer of ≥1/64
is diagnostic. Bacterial DNA can be detected from blood, skin biopsy
and from ticks with the molecular methods [2,4]. Gene loci specific
to Rickettsia genus 17-kDa lipoprotein, citrate synthase, OmpB
(outermembrane protein B) and locus specific to spotted fever,
OmpA (outer membrane protein A) could be amplified by PCR [11-
15]. According to ESCAR study group diagnostic criteria (ESCMID
Study Group for Coxiella, Anaplasma, Rickettsia and Bartonella) a
score over 25 is diagnostic [16,17].
In our case, patient was admitted to the clinic with the presumptive
diagnosis of CCHF. As the testing results were negative for CCHF,
the patient was evaluated for other infectious diseases including
Ricketsiosis and Q fever because of the history of dog feeding, escar
presence and found positive for R. conorii antibodies indicating the
fact that Rickestiosis could be easily misdiagnosed by CCHF and the
prevalence is definitely underestimated due to the lack of testing for
Rickettsial diseases in Turkey.
R. conorii IgG seroprevalence in Turkey show variation up
to 36.8% [6]. In Turkey, MSF case reports are rare and generally
localized from Thrace Region, near the border of Greece where
Rickettsiosis is endemic [18-20]. In surveillance studies, R. conorii
antibodies were detected in North Greece [21] with a frequency of
7.9%. It is estimated that there are at least seven times more cases than
reported cases [19,15].
Although Rhipicephalus sanguineus is well-adapted to the urban
environment, it is specific to the host and fed from human rarely.
They fed from human in an environment with the lack of dog host
and repellent use of the dogs [17]. R. conorii proliferate in almost
all organs, especially salivary glands, and allow Rickettsia spp. to be
transmitted to vertebrate hosts during feeding [6]. In recent years
there has been an increase in the number of cases. The increase in
the incidence could be explained with increase in the number of
ticks, frequency of the contact of the human with infected ticks due
to the more often outdoor activities. In addition, climate changes
such as temperature increase, rainfall reduction, and a decrease in the
number of frosty days in the previous year have also caused changes
in tick activity [11].
The MSF case with mortality includes similar epidemiological
features, clinical symptoms and laboratory findings with fatal CCHF
[7]. In addition, some studies pointed to the co-existence of MSF
and CCHF [6]. In a study, of the serum samples collected for CCHF
screening, 36.8% were positive for Rickettsia, 7.6% for both CCHF
and Rickettsia IgG [6]. In Albania, of the CCHF suspected cases final
diagnosis can be achieved in 82.2% of the cases with the distribution
of 38.2% CCHF, 11.7% Hantavirus, 29.4% leptospirosis, and 2.9%
Ricketsiosis [9]. It is clear that CCHF should be considered in the
differential diagnosis due to the identical epidemiological, clinical
and laboratory findings. Lack of escar and tick-bite history may
lead to misdiagnosis ve rash may be considered primarily as allergy
[7]. Additionally rubella, rubeola, leptospirosis, syphilis, infectious
mononucleosis, ehrlichosis, anaplasmosis, allergic reactions, drug
eruptions should be kept in mind [7]).
Among ticks removed from patients located in Istanbul half of
the ticks were positive for Rickettsia spp. by PCR and R. conorii was
present in the 4% of the Rh. bursa [3]. Rh. sangineus is not the only
vector for R. conorii and Rh. bursa may be detected [1]. Moreover,
R. conorii was detected from Rh. bursa removed from MSF cases. R.
helvetica and R. monacensis, previously related with MSF-like disease
were also detected from I. ricinus ticks [10].
Conclusion
In conclusion, it should be considered that Turkey is endemic for tick borne infections due to the geographical location and tick diversity. MSF should be included in the differential diagnosis of fever, headache, myalgia even in the absence of escar and rash during spring and summer, exposure to ticks, dog feeding history. This report is presented at the III. KLIMUD Congress orally (18-22 November 2015, Antalya).
Funding
This study is supported by internal funding.
References
- Parola P, Paddock CD, Socolovschi C, Labruna MB, Mediannikov O, Kernif T, et al. Update on tick-borne rickettsioses around the world: a geographic approach. Clin Microbiol Rev. 2013; 26: 657-702.
- Parola P, Paddock CD, Raoult D. Tick-borne rickettsioses around the world: emerging diseases challenging old concepts. Clin Microbiol Rev. 2005; 18: 719-756.
- Gargili A, Palomar AM, Midilli K, Portillo A, Kar S, Oteo JA. Rickettsia species in ticks removed from humans in Istanbul, Turkey. Vector Borne Zoonotic Dis. 2012; 12: 938-41.
- Raoult D, Tissot-Dupont H, Caraco P, Brouqui P, Drancourt M, Charrel C. Mediterranean spotted fever in Marseille: descriptive epidemiology and the influence of climatic factors. Eur J Epidemiol. 1992; 8: 192-197.
- Kuloglu F, Rolain JM, Akata F, Eroglu C, Celik AD, Parola P. Mediterranean spotted fever in the Trakya region of Turkey. Ticks Tick Borne Dis. 2012; 3: 298-304.
- Günes T, Poyraz Ö, Atas M, Turgut NH. The seroprevalence of Rickettsia conorii in humans living in villages of Tokat Province in Turkey, where Crimean-Congo hemorrhagic fever virus is endemic, and epidemiological similarities of both infectious agents. Turk J Med Sci. 2012; 42: 441-448.
- Papa A, Dalla V, Petala A, Maltezou HC, Maltezos E. Fatal Mediterranean spotted fever in Greece. Clin Microbiol Infect. 2010; 16: 589-592.
- Antón E, Font B, Muñoz T, Sanfeliu I, Segura F. Clinical and laboratory characteristics of 144 patients with mediterranean spotted fever. Eur J Clin Microbiol Infect Dis. 2003; 22: 126-128.
- Papa A, Bino S, Papadimitriou E, Velo E, Dhimolea M, Antoniadis A. Suspected Crimean Congo Haemorrhagic Fever cases in Albania. Scand J Infect Dis. 2008; 40: 978-980.
- Oteo JA, Portillo A. Tick-borne rickettsioses in Europe. Ticks Tick Borne Dis. 2012; 3: 271-278.
- Rovery C, Brouqui P, Raoult D. Questions on Mediterranean spotted fever a century after its discovery. Emerg Infect Dis. 2008; 14: 1360-1367.
- Brouqui P, Parola P, Fournier PE, Raoult D. Spotted fever rickettsioses in southern and Eastern Europe. FEMS Immunol Med Microbiol. 2007; 49: 2-12.
- Rovery C, Raoult D. Mediterranean spotted fever. Infect Dis Clin North Am. 2008; 22: 515-30.
- La Scola B, Raoult D. Laboratory diagnosis of rickettsioses: current approaches to the diagnosis of old and new rickettsial diseases. J Clin Microbiol. 1997; 35: 2715–2727.
- Raoult D, Weiller PJ, Chagnon A, Chaudet H, Gallais H, Casanova P. Mediterranean spotted fever: clinical, laboratory and Epidemiological features of 199 cases. Am J Trop Med Hyg. 1986; 35: 845-50.
- Brouqui P, Bacellar F, Baranton G, Birtles RJ, Bjoërsdorff A, Blanco JR, et al. Guidelines for the diagnosis of tick-borne bacterial diseases in Europe. Clin Microbiol Infect. 2004; 10: 1108-32.
- Parola P. Tick-borne rickettsial diseases: emerging risks in Europe. Comp Immunol Microbiol Infect Dis. 2004; 27: 297-304.
- Kuloglu F, Rolain JM, Fournier PE, Akata F, Tugrul M, Raoult D. First isolation of Rickettsia conorii from humans in the Trakya (European) region of Turkey. Eur J Clin Microbiol Infect Dis. 2004; 23: 609-14.
- de Sousa R, Nóbrega SD, Bacellar F, Torgal J. Mediterranean spotted fever in Portugal: risk factors for fatal outcome in 105 hospitalized patients. Ann N Y Acad Sci. 2003; 990: 285-294.
- Vural T, Ergin C, Sayin F. Investigation of Rickettsia conorii antibodies in the Antalya area. Infection. 1998; 26: 170-172.
- Daniel SA, Manika K, Arvanmdou M, Antoniadis A. Prevalence of Rickettsia conorii and Rickettsia typhi infections in the population of northern Greece. Am J Trop Med Hyg. 2002; 66: 76-79.