Case Report
A Case Report of Hairy Cell Leukemia: An Unusual Presentation
Khai Li Chai1*, Thomas Morris2 and Ali Bazargan1
1Department of Hematology, St Vincent’s Hospital, Melbourne, Australia
2Department of Neurosurgery, St Vincent’s Private Hospital, Melbourne, Australia
*Corresponding author: Ali Bazargan, St Vincent’s Hospital Melbourne, 41 Victoria Parade, Fitzroy Victoria 3065, Australia
Published: 08 Aug, 2017
Cite this article as: Chai KL, Morris T, Bazargan A. A Case
Report of Hairy Cell Leukemia: An
Unusual Presentation. Ann Clin Case
Rep. 2017; 2: 1412.
Abstract
Hairy cell leukemia is a rare B cell lymphoproliferative disorder. Patients typically present with peripheral cytopenias, circulating leukemia cells, marked splenomegaly and marrow infiltration. We describe a case of a 68 year old man who presented solely with psychiatric symptoms of delusions, without the classical features of cytopenias or splenomegaly. Initial imaging revealed involvement with lymphoma at multiple nodal and extranodal sites, including the base of skull, skeletal bone, bone marrow and pleura. Histology of the lesions from the base of skull, bone marrow and iliac bone revealed small B cells with the typical immunophenotype for hairy cell leukemia, but with aberrant expression of cyclin D1. Only rare cases of skeletal bone disease and neurological involvement have been previously reported in the literature. He achieved only partial response to initial treatment with cladribine, and subsequently achieved complete metabolic response following treatment with rituximab. He experienced no toxicities with treatment and remains in complete remission at 12 months following treatment. This case highlights that careful hematopathological assessment and clinical correlation is required for accurate diagnosis of unusual cases of hairy cell leukemia. In addition, this case also demonstrates that a combination of cladribine and rituximab therapy can be effective in deepening frontline response in patients with hairy cell leukemia without significant treatment-related toxicity.
Introduction
Hairy Cell Leukemia (HCL) is a rare B cell lymphoproliferative disorder, with patients typically
presenting with cytopenias, marked splenomegaly and circulating leukemia cells. Leukemic cells
classically have central nuclei, abundant cytoplasm with hairy-like projections, and express CD11c,
CD25, CD103, CD123, CD20, CD22, CD52, and mild to moderate expression of cyclin D1 [1].
Rare cases of neurological and skeletal bone involvement have been reported in the literature
[2-4]. We describe a case of a 68 year old man diagnosed with HCL involving base of skull, skeletal bone and bone marrow, but without classical features of cytopenias or splenomegaly.
Case Presentation
A 68 year old man with a longstanding history of paranoid personality disorder presented with
acute delusional symptoms. His past history was significant for atrial fibrillation, type 2 diabetes
mellitus, dyslipidemia and paranoid personality disorder. His medications included rivaroxaban,
digoxin, perindopril and simvastatin. He did not have any B symptoms or other neurological
symptoms or signs. On further questioning, there was no family history of dementia or malignancy.
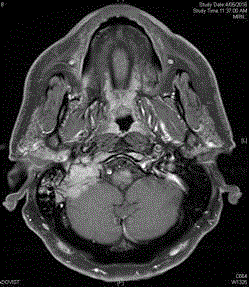
Brain CT and MRI scans (Figure 1) showed a soft tissue mass in the right skull base involving the right occipital bone and petrous temporal bone with extension into the posterior fossa, with an
enhancing component of the mass extending along the right glossopharyngeal nerve into the right
jugular foramen and becoming contiguous with a large soft tissue mass within the suboccipital region,
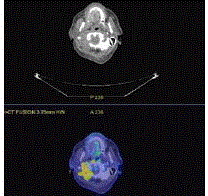
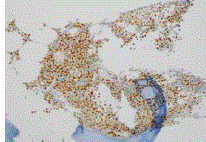
measuring 4.7 cm x 2.3 cm x 5.3 cm in size. A staging PET scan (Figure 2) showed moderate FDG avidity at the right base of skull, bony involvement at the pelvis and right humerus, pleural uptake,
and nodal uptake within the anterior mediastinum and right internal mammary chain. Full blood
count showed mild thrombocytopenia of 143x10^9/L and a low monocyte count of 0.1x10^9/L.
There were no circulating hairy cells seen on blood film. Peripheral blood flow cytometry did not
detect any monoclonal B cell population. Cerebrospinal fluid analysis revealed elevated protein
levels (0.91 g/L). Flow cytometry of CSF did not detect any monoclonal B cell population.
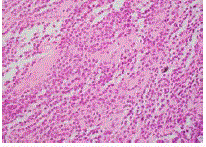
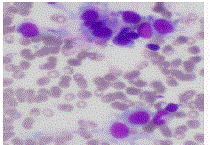
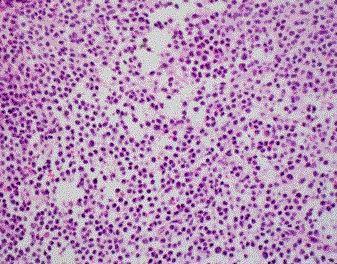
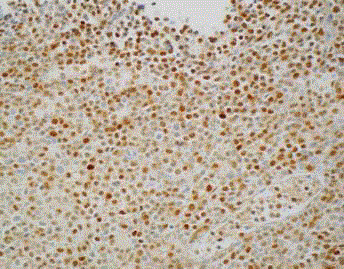
Initial biopsy of the right base of skull lesion (Figures 3-5)
showed infiltration of small cells with regular round nuclei, which
on immunohistochemistry were consistent with B cells that were
CD5-, CD20+, CD138-, cyclin D1+ diffuse, CD10+, BCL2+ strong
diffuse, BCL-6 weak, CD25+, DBA44+, MYC- and EBER-ISH-. Due
to the high cyclin D1 expression, an initial diagnosis of mantle cell
lymphoma was considered. However, the findings were inconsistent
with classic mantle cell lymphoma given the negativity of CD5
expression on immunohistochemistry and the low Ki67 index.
Further testing with FISH was negative for t (11;14).
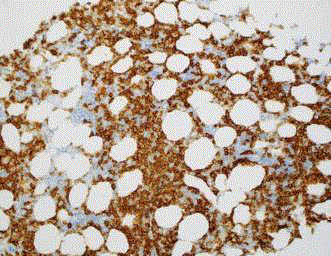
A staging bone marrow biopsy from the right posterior
superior iliac spine (Figures 6-9) showed a normocellular marrow
with involvement with hairy cells morphologically and on flow
cytometry. Flow cytometric analysis showed 15% of lymphocytes
were B cells of hairy cell phenotype (CD5-, CD 10 -, CD11c+, CD
19+, CD20+, CD22+, CD23-, CD25+, CD103+), and monoclonality
was demonstrated by light chain restriction. Bone marrow trephine
showed diffuse effacement of the marrow space with sheets of small lymphoid cells and scant pale cytoplasm. These cells were CD20+, BCL-2+, BCL-6+ weak, CD10+ patchy, and once again had diffuse
cyclin D1 expression. FISH once again did not detect t(11;14).
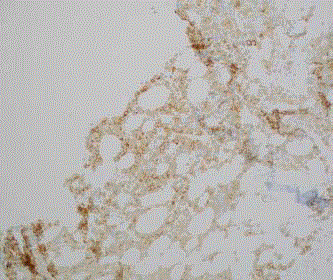
Subsequent repeat PET-guided iliac bone biopsy on the contralateral
side (Figures 10 and 11) validated the same findings on the bone marrow
biopsy.
Given classic morphological and immunophenotypical features
of HCL along with negative FISH for t(11;14), he was diagnosed with
HCL involving multiple nodal and extranodal sites, including base of
skull, skeletal bone, bone marrow, and pleura. Following diagnosis
of HCL, treatment was commenced with a standard course of
intravenous cladribine. Restaging PET scan post cladribine showed persistent mild-moderate FDG avidity at nodal and extranodal
sites consistent with partial metabolic response. Repeat bone
marrow biopsy showed marked reduction of HCL with no hairy
cells detected by flow cytometry and small amounts of DBA44+ B
cells on the trephine. BRAF V600E mutation was not detected on
this sample, which is likely due to low tumor burden from this site.
Unfortunately, BRAF V600E mutation testing was not performed on
the initial diagnostic sample. Given the lack of complete response
post cladribine, he was commenced on four cycles of rituximab of
375 mg/m2. There were no toxicities from treatment. Subsequently,
his full blood count normalized and a further restaging PET scan was consistent with complete metabolic response. Currently, he remains in complete remission at 12 months post treatment with resolution
of delusions.
There have been no randomized controlled trials to outline the
optimum approach of combined chemoimmunotherapy in HCL.
Most guidelines for frontline treatment of HCL recommend use
of purine analogues, in particular cladribine or pentostatin, and
rituximab is not routinely incorporated into this regimen. However,
failure to achieve complete remission to purine analogue therapy has
now been recognized as a poor prognostic factor in classical hairy
cell leukemia [5]; and some studies have demonstrated the efficacy of
rituximab therapy in HCL, both in patients with incomplete response
in frontline therapy, and in relapsed or refractory settings [6-8].
Figure 1
Figure 2
Figure 3
Figure 4
Figure 5
Figure 6
Figure 7
Figure 8
Figure 9
Figure 10
Figure 11
Discussion
This is an unusual case of HCL due to several reasons. Firstly,
HCL rarely involves skeletal bone, lung pleura and the central nervous
system. While bone marrow is involved in almost all cases of HCL,
bone involvement with radiographic evidence of osteolytic lesions is
rare. Reports of skeletal bone involvement have been found in 3% of
cases [3,9]. Reported cases show that bony lesions can affect multiple
sites, but most commonly proximal long bones, and is almost always
associated with pain and extensive involvement of the bone marrow
[10]. These lesions are often lytic, but cases of diffuse osteosclerosis,
mixed sclerotic and lytic pattern, and osteoporosis have also been
reported [9]. Our patient differs to these cases due to the predominant
location of the lesions at the skull base, and without symptoms from
bone pain. Secondly, it is very unusual for HCL to involve the central
nervous system. Descriptions of nervous system involvement in HCL
are largely limited to case reports, which have documented a range of
neurological symptoms. These include isolated radicular infiltration,
intracerebral hemorrhage secondary to leukostasis, intraparenchymal
brain involvement, and tumor invasion of the cord with compression
resulting from tumor infiltration of vertebrae [2,11]. A review of 108
patients in the Mayo Clinic with HCL found only 5 patients who had
neurological symptoms. Of these, one had leukemic compression of
neural structures, three had central nervous system infections and one
had polyradiculopathy [12]. None were reported to have enhancing
lesions within the cranial nerve distribution, or to have presented with
personality change or paranoid delusions, as was the case with our
patient. Thirdly, HCL frequently presents with peripheral cytopenias
and splenomegaly, which was not seen in this case. In a recent review,
the incidence of HCL without splenomegaly is thought to be between
0-40%, and the incidence of HCL without cytopenias is thought to be
less than 25% [13,14]. In a recent analysis of over 500 patients, anemia
defined as a hemoglobin level less than 120 g/L was seen in 75–84%
of patients at presentation, platelets less than 100 000/ml were seen in
57–79% of patients at presentation, and leucopenia was seen in 60%
of patients [14,15].
Lastly, the diffuse expression of Cyclin D1 as seen in the biopsies of
the base of skull lesion, bone marrow biopsy and iliac bone biopsy, is
unusual for HCL. Cyclin D1 is a cell cycle protein that is overexpressed
most frequently in mantle cell lymphoma due to t(11;14)(q13;q32),
which involves translocation of the immunoglobulin heavy chain
gene on chromosome 14 and the bcl-1 region on chromosome 11.
While many cases of HCL can have cyclin D1 overexpression, the
level of expression is usually mild to moderate and usually involves
a subset of the lymphoma cells, as compared to the high and diffuse
level of expression typically seen in mantle cell lymphoma [16]. Cyclin D1 overexpression has also been reported in head and neck, breast
and lung carcinomas, as well as other lymphoproliferative disorders,
including rare cases of CLL, lymphoplasmacytic lymphoma and
multiple myeloma [16].
Conclusion
This case illustrates an unusual presentation of HCL, involving multiple atypical sites, with over expression of cyclin D1, and without the classical involvement of peripheral blood or spleen. In this patient, due to the unusual clinical presentation, disease distribution and aberrant immunohistochemistry staining, an initial diagnosis of mantle cell lymphoma was considered. Subsequent testing with FISH and further biopsies were required to confirm the diagnosis of hairy cell leukemia. This case highlights that accurate diagnoses often require careful assessment of morphology, immunophenotypic and cytogenetic findings in order to deliver appropriate treatment and prognostication. In this case, the patient failed to achieve complete response to initial treatment with cladribine, but went into complete remission with further administration of rituximab. As such, this case also demonstrates that rituximab therapy can be effective in deepening frontline response to cladribine in HCL which may be important in optimizing progression-free survival in patients with HCL without the addition of significant treatment-related toxicity.
References
- Grever MR. How I treat hairy cell leukemia. Blood. 2010; 115: 21-28.
- Chandana SR, Kotecha R, Al-Janadi A, Chang HT, Conley BA. Rare case of hairy cell leukemia with brain parenchymal involvement: a diagnostic dilemma. J Clin Oncol. 2013; 31: e186-e8.
- Yonal-Hindilerden I, Hindilerden F, Bulut-Dereli S, Yıldız E, Dogan IO, Nalcaci M. Hairy Cell Leukemia Presenting with Isolated Skeletal Involvement Successfully Treated by Radiation Therapy and Cladribine: A Case Report and Review of the Literature. Case Rep Hematol. 2015; 2015: 803921.
- Lal A, Tallman MS, Soble MB, Golubovich I, Peterson L. Hairy cell leukemia presenting as localized skeletal involvement. Leuk Lymphoma. 2002; 43: 2207-11.
- Jones G, Parry-Jones N, Wilkins B, Else M, Catovsky D; British Committee for Standards in Haematology. Revised guidelines for the diagnosis and management of hairy cell leukaemia and hairy cell leukaemia variant*. Br J Haematol. 2012; 156: 186-195.
- Ravandi F, O'Brien S, Jorgensen J, Pierce S, Faderl S, Ferrajoli A, et al. Phase 2 study of cladribine followed by rituximab in patients with hairy cell leukemia. Blood. 2011; 118: 3818-3823.
- Ravandi F, Jorgensen JL, O'Brien SM, Verstovsek S, Koller CA, Faderl S, et al. Eradication of minimal residual disease in hairy cell leukemia. Blood. 2006; 107: 4658-4662.
- Else M, Osuji N, Forconi F, Dearden C, Del Giudice I, Matutes E, et al. The role of rituximab in combination with pentostatin or cladribine for the treatment of recurrent/refractory hairy cell leukemia. Cancer. 2007; 110: 2240-2247.
- Tadmor T, Polliack A. Unusual clinical manifestations, rare sites of involvement, and the association of other disorders with hairy cell leukemia. Leuk Lymphoma. 2011; 52: 57-61.
- Demanes DJ, Lane N, Beckstead JH. Bone involvement in hairy-cell leukemia. Cancer. 1982; 49: 1697-701.
- Chaudhry MS, Macdonald D, Strickland N. Hairy cell leukemia presenting as a cranial mass. Am J Hematol. 2011; 86: 423-424.
- Kimmel DW, Hermann RC Jr, O'Neill BP. Neurologic complications of hairy cell leukemia. Arch Neurol. 1984; 41: 202-203.
- Venkatesan S, Purohit A, Aggarwal M, Manivannan P, Tyagi S, Mahapatra M, et al. Unusual presentation of hairy cell leukemia: a case series of four clinically unsuspected cases. Indian J Hematol Blood Transfus. 2014; 30: 413-417.
- Kraut EH. Clinical manifestations and infectious complications of hairy-cell leukemia. Best Pract Res Clin Haematol. 2003; 16: 33-40.
- Jansen J, Hermans J, For The Collaborative Study G. Splenectomy in hairy cell leukemia: A retrospective multicenter analysis. Cancer. 1981; 47: 2066-2076.
- Miranda RN, Briggs RC, Kinney MC, Veno PA, Hammer RD, Cousar JB. Immunohistochemical detection of cyclin D1 using optimized conditions is highly specific for mantle cell lymphoma and hairy cell leukemia. Modern Pathology. 2000; 13: 1308-1314.